

Exhibit 99.2 MM120 for GAD Investor Presentation March 2024

Disclaimer This presentation (the “Presentation”) has been prepared by Mind Medicine (MindMed) Inc. (“MindMed”, the “Company”, “we”, “our” or “us) solely for informational purposes. None of MindMed, its affiliates or any of their respective employees, directors, officers, contractors, advisors, members, successors, representatives or agents makes any representation or warranty as to the accuracy or completeness of any information contained in this Presentation and shall have no liability for any representations (expressed or implied) contained in, or for any omissions from, this Presentation. This Presentation does not constitute an offering of, or a solicitation of an offer to purchase, securities of MindMed and under no circumstances is it to be construed as a prospectus or advertisement or public offering of securities. Any trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of the products or services of MindMed. Any amounts are in USD unless otherwise noted. MindMed’s securities have not been approved or disapproved by the Securities and Exchange Commission (the SEC ) or by any state, provincial or other securities regulatory authority, nor has the SEC or any state, provincial or other securities regulatory authority passed on the accuracy or adequacy of this Presentation. Any representation to the contrary is a criminal offense. Cautionary Note Regarding Forward-Looking Statements This Presentation contains, and our officers and representatives may from time to time make, “forward-looking statements” within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995 and other applicable securities laws. Forward-looking statements can often, but not always, be identified by words such as “plans”, “expects”, “is expected”, “budget”, “scheduled”, “estimates”, “forecasts”, “intends”, “anticipates”, will”, “projects”, or “believes” or variations (including negative variations) of such words and phrases, or statements that certain actions, events, results or conditions “may”, “could”, “would”, “might” or “will” be taken, occur or be achieved, and similar references to future periods. Except for statements of historical fact, examples of forward-looking statements include, among others, statements pertaining to: the development and commercialization of any medicine or treatment, or the efficacy of either of the foregoing, the success and timing of our development activities; the success and timing of our planned clinical trials; our ability to meet the milestones set forth herein; the likelihood of success of any clinical trials or of obtaining FDA or other regulatory approvals; our cash runway funding operations through key clinical readouts and into 2026; the likelihood of obtaining patents or the efficacy of such patents once granted and the potential for the markets that MindMed is anticipating to access. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the economy and other future conditions as of the date of this Presentation. While MindMed considers these assumptions to be reasonable, the assumptions are inherently subject to significant business, social, economic, political, regulatory, competitive and other risks and uncertainties that are difficult to predict and many of which are outside of MindMed’s control, and actual results and financial condition may differ materially from those indicated in the forward- looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause actual results and financial condition to differ materially from those indicated in the forward-looking statements include, among others, the following: our ability to raise capital to complete its plans and fund its studies; the medical and commercial viability of the contemplated medicines and treatments being developed; MindMed’s history of negative cash flows; MindMed’s limited operating history; incurrence of future losses; compliance with laws and regulations; difficulty associated with research and development; risks associated with clinical trials or studies; heightened regulatory scrutiny; early stage product development; clinical trial risks; regulatory approval processes; novelty of the psychedelic inspired medicines industry; as well as those risk factors discussed or referred to throughout the “Risk Factors” sections of MindMed’s most recently filed Annual Report on Form 10-K filed with the SEC and in other filings we make in the future with the SEC and the securities regulatory authorities in all provinces and territories of Canada, available under the Company’s profile on SEDAR at www.sedar.com. Any forward-looking statement made by MindMed in this Presentation is based only on information currently available to the Company and speaks only as of the date on which it is made. MindMed undertakes no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise. Cautionary Note Regarding Regulatory Matters The United States federal government regulates drugs through the Controlled Substances Act. MM120 is a proprietary, pharmaceutically optimized form of lysergide D-tartrate and MM402, or R(-)-MDMA, is our proprietary form of the R-enantiomer of MDMA (3,4-methylenedioxymethamphetamine). Lysergide and MDMA are Schedule I substances under the Controlled Substances Act. While the Company is focused on programs using psychedelic or hallucinogenic compounds and non-hallucinogenic derivatives of these compounds, including in its MM120, MM402 and other product candidates, the Company does not have any direct or indirect involvement with the illegal selling, production or distribution of any substances in the jurisdictions in which it operates. The Company is a neuro-pharmaceutical drug development company and does not deal with psychedelic or hallucinogenic substances except within laboratory and clinical trial settings conducted within approved regulatory frameworks. The Company's products will not be commercialized prior to applicable regulatory approval, which will only be granted if clinical evidence of safety and efficacy for the intended uses is successfully developed. Market and Industry Data This Presentation includes market and industry data that has been obtained from third party sources, including industry publications. MindMed believes that the industry data is accurate and that the estimates and assumptions are reasonable, but there is no assurance as to the accuracy or completeness of this data. Third party sources generally state that the information contained therein has been obtained from sources believed to be reliable, but there is no assurance as to the accuracy or completeness of included information. Although the data is believed to be reliable, MindMed has not independently verified any of the data from third party sources referred to in this Presentation or ascertained the underlying economic assumptions relied upon by such sources. References in this Presentation to research reports or to articles and publications should be not construed as depicting the complete findings of the entire referenced report or article. MindMed does not make any representation as to the accuracy of such information. Investor Presentation | March 2024 2

Introductory Remarks Robert Barrow Chief Executive Officer
We Aim To Be A Global Leader In Brain Health Pipeline Management Diversified pipeline of clinical Expertise in drug programs targeting development and significant unmet medical needs commercialization Research Expected Runway Leveraging decades of preclinical and Expected cash runway through key clinical research with promising results clinical readouts and into 2026* in Phase 2b Market Protection Strategies IP and R&D strategies intended to maximize market exclusivity and protection *The company’s cash and cash equivalents of $99.7 million as of December 31, 2023 and committed credit facility are expected to fund operations into 2026. Investor Presentation | March 2024 5
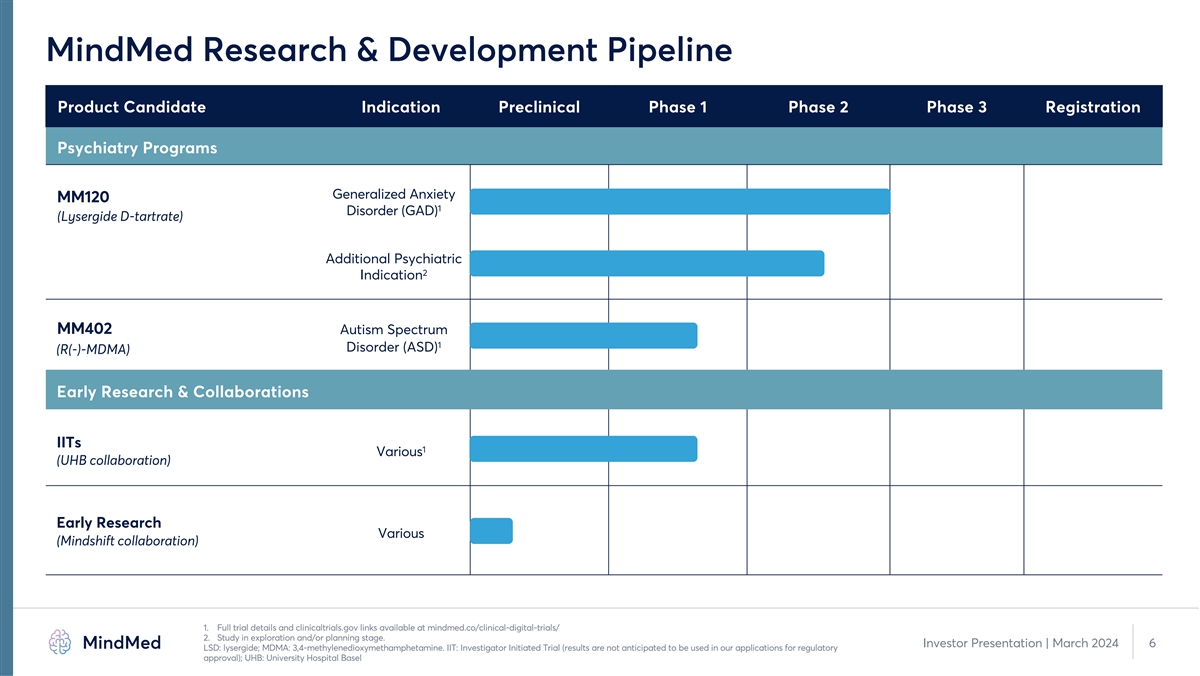
MindMed Research & Development Pipeline Product Candidate Indication Preclinical Phase 1 Phase 2 Phase 3 Registration Psychiatry Programs Generalized Anxiety MM120 1 Disorder (GAD) (Lysergide D-tartrate) Additional Psychiatric 2 Indication MM402 Autism Spectrum 1 Disorder (ASD) (R(-)-MDMA) Early Research & Collaborations IITs 1 Various (UHB collaboration) Early Research Various (Mindshift collaboration) 1. Full trial details and clinicaltrials.gov links available at mindmed.co/clinical-digital-trials/ 2. Study in exploration and/or planning stage. Investor Presentation | March 2024 6 LSD: lysergide; MDMA: 3,4-methylenedioxymethamphetamine. IIT: Investigator Initiated Trial (results are not anticipated to be used in our applications for regulatory approval); UHB: University Hospital Basel
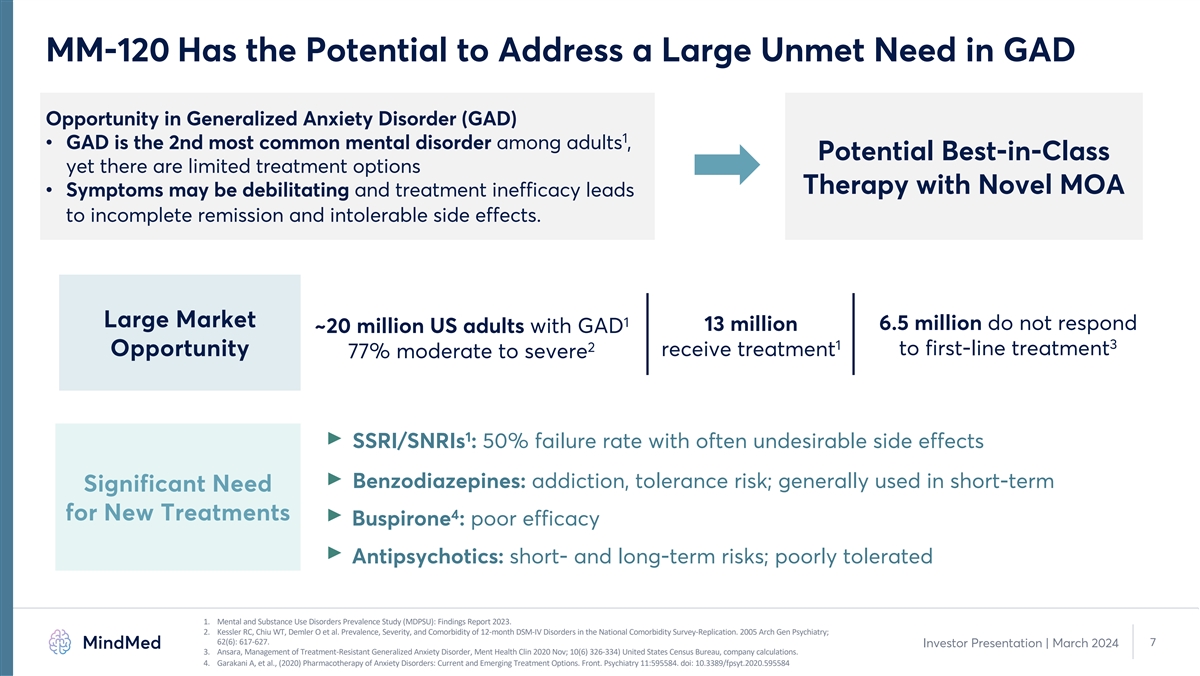
MM-120 Has the Potential to Address a Large Unmet Need in GAD Opportunity in Generalized Anxiety Disorder (GAD) 1 • GAD is the 2nd most common mental disorder among adults , Potential Best-in-Class yet there are limited treatment options Therapy with Novel MOA • Symptoms may be debilitating and treatment inefficacy leads to incomplete remission and intolerable side effects. 1 Large Market 13 million 6.5 million do not respond ~20 million US adults with GAD 3 1 2 to first-line treatment Opportunity receive treatment 77% moderate to severe 1 SSRI/SNRIs : 50% failure rate with often undesirable side effects Benzodiazepines: addiction, tolerance risk; generally used in short-term Significant Need 4 for New Treatments Buspirone : poor efficacy Antipsychotics: short- and long-term risks; poorly tolerated 1. Mental and Substance Use Disorders Prevalence Study (MDPSU): Findings Report 2023. 2. Kessler RC, Chiu WT, Demler O et al. Prevalence, Severity, and Comorbidity of 12-month DSM-IV Disorders in the National Comorbidity Survey-Replication. 2005 Arch Gen Psychiatry; 62(6): 617-627. 7 Investor Presentation | March 2024 3. Ansara, Management of Treatment-Resistant Generalized Anxiety Disorder, Ment Health Clin 2020 Nov; 10(6) 326-334) United States Census Bureau, company calculations. 4. Garakani A, et al., (2020) Pharmacotherapy of Anxiety Disorders: Current and Emerging Treatment Options. Front. Psychiatry 11:595584. doi: 10.3389/fpsyt.2020.595584
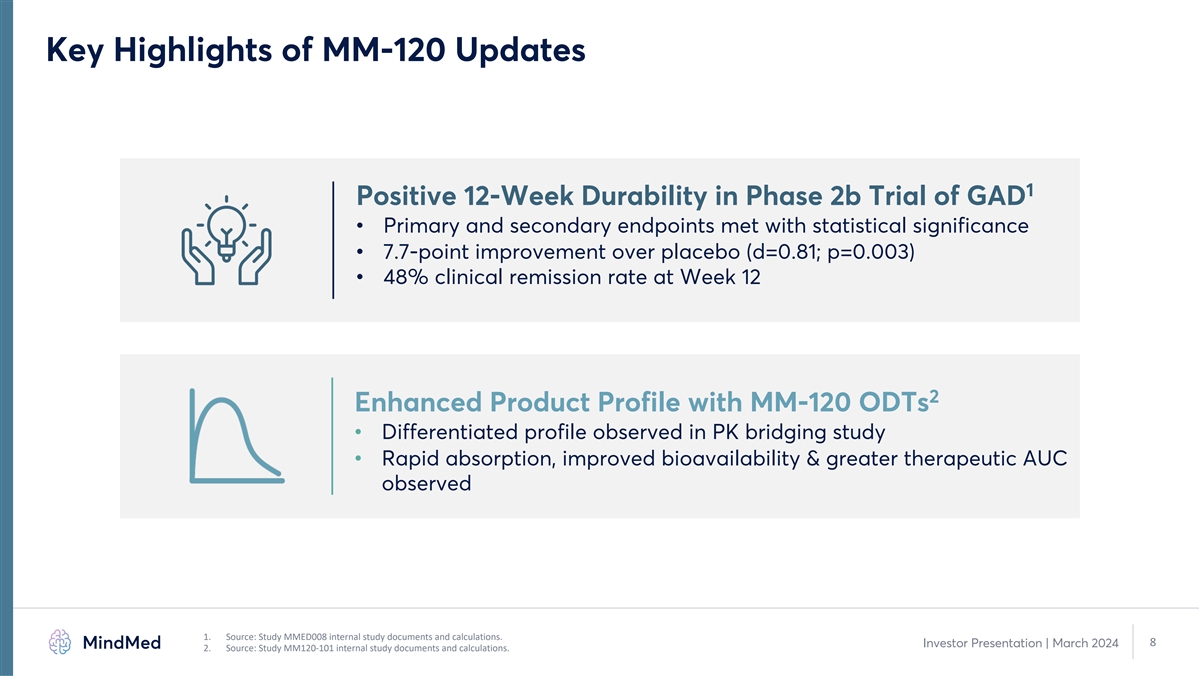
Key Highlights of MM-120 Updates 1 Positive 12-Week Durability in Phase 2b Trial of GAD • Primary and secondary endpoints met with statistical significance • 7.7-point improvement over placebo (d=0.81; p=0.003) • 48% clinical remission rate at Week 12 2 Enhanced Product Profile with MM-120 ODTs • Differentiated profile observed in PK bridging study • Rapid absorption, improved bioavailability & greater therapeutic AUC observed 1. Source: Study MMED008 internal study documents and calculations. 8 Investor Presentation | March 2024 2. Source: Study MM120-101 internal study documents and calculations.
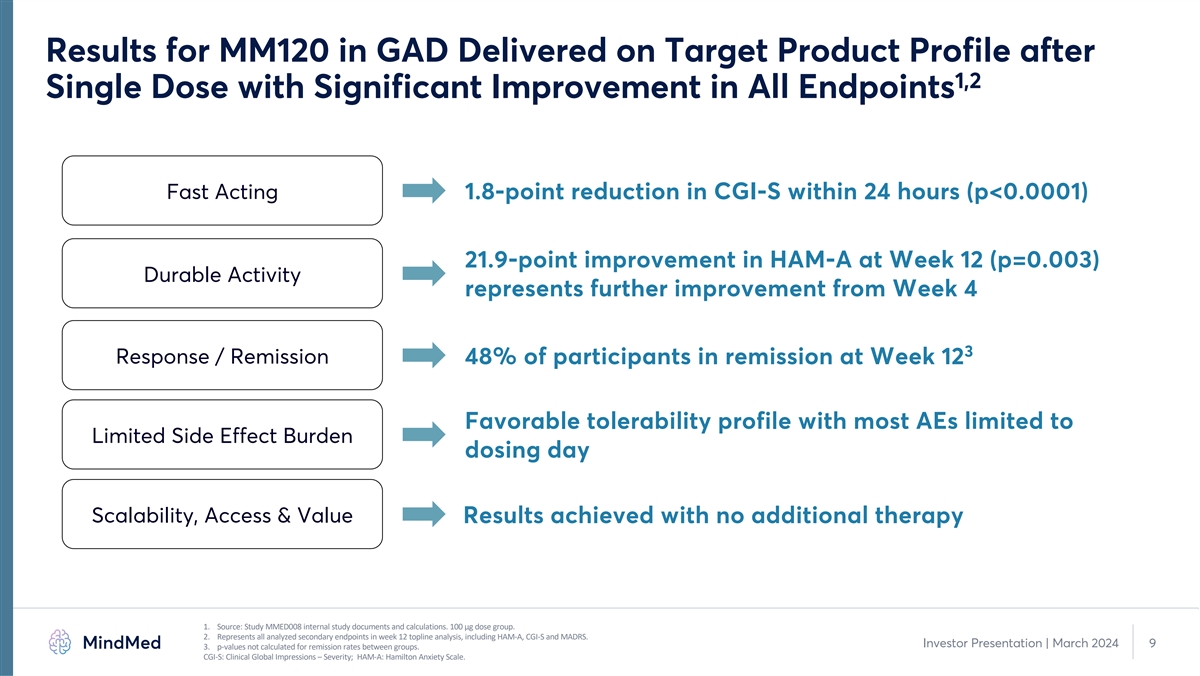
Results for MM120 in GAD Delivered on Target Product Profile after 1,2 Single Dose with Significant Improvement in All Endpoints Fast Acting 1.8-point reduction in CGI-S within 24 hours (p<0.0001) 21.9-point improvement in HAM-A at Week 12 (p=0.003) Durable Activity represents further improvement from Week 4 3 Response / Remission 48% of participants in remission at Week 12 Favorable tolerability profile with most AEs limited to Limited Side Effect Burden dosing day Scalability, Access & Value Results achieved with no additional therapy 1. Source: Study MMED008 internal study documents and calculations. 100 µg dose group. 2. Represents all analyzed secondary endpoints in week 12 topline analysis, including HAM-A, CGI-S and MADRS. Investor Presentation | March 2024 9 3. p-values not calculated for remission rates between groups. CGI-S: Clinical Global Impressions – Severity; HAM-A: Hamilton Anxiety Scale.
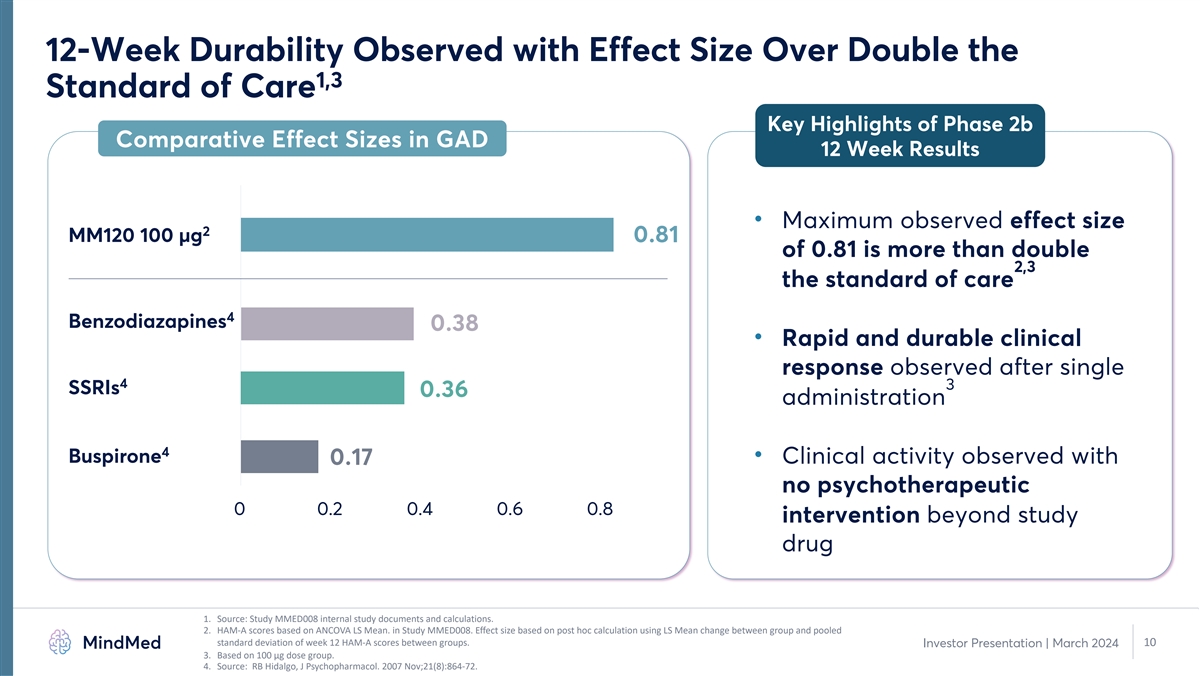
12-Week Durability Observed with Effect Size Over Double the 1,3 Standard of Care Key Highlights of Phase 2b Comparative Effect Sizes in GAD 12 Week Results • Maximum observed effect size 2 MM120 100 µg 0.81 of 0.81 is more than double 2,3 the standard of care 4 Benzodiazapines 0.38 • Rapid and durable clinical response observed after single 4 3 SSRIs 0.36 administration 4 Buspirone • Clinical activity observed with 0.17 no psychotherapeutic 0 0.2 0.4 0.6 0.8 intervention beyond study drug 1. Source: Study MMED008 internal study documents and calculations. 2. HAM-A scores based on ANCOVA LS Mean. in Study MMED008. Effect size based on post hoc calculation using LS Mean change between group and pooled standard deviation of week 12 HAM-A scores between groups. 10 Investor Presentation | March 2024 3. Based on 100 µg dose group. 4. Source: RB Hidalgo, J Psychopharmacol. 2007 Nov;21(8):864-72.
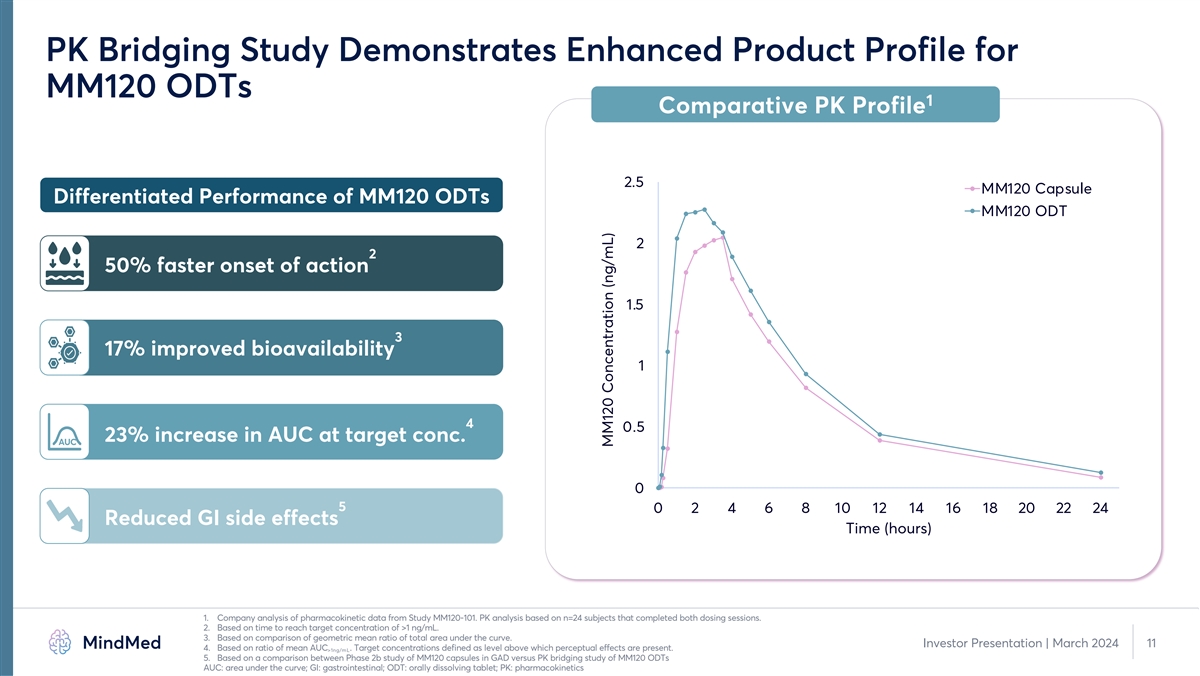
PK Bridging Study Demonstrates Enhanced Product Profile for MM120 ODTs 1 Comparative PK Profile 2.5 MM120 Capsule Differentiated Performance of MM120 ODTs MM120 ODT 2 2 50% faster onset of action 1.5 3 17% improved bioavailability 1 4 0.5 23% increase in AUC at target conc. AUC 0 5 0 2 4 6 8 10 12 14 16 18 20 22 24 Reduced GI side effects Time (hours) 1. Company analysis of pharmacokinetic data from Study MM120-101. PK analysis based on n=24 subjects that completed both dosing sessions. 2. Based on time to reach target concentration of >1 ng/mL. 3. Based on comparison of geometric mean ratio of total area under the curve. Investor Presentation | March 2024 11 4. Based on ratio of mean AUC . Target concentrations defined as level above which perceptual effects are present. >1ng/mL 5. Based on a comparison between Phase 2b study of MM120 capsules in GAD versus PK bridging study of MM120 ODTs AUC: area under the curve; GI: gastrointestinal; ODT: orally dissolving tablet; PK: pharmacokinetics MM120 Concentration (ng/mL)

MM120 LSD-D-tartrate for Generalized Anxiety Disorder (GAD) Summary of Full Topline Results from Phase 2b Trial Daniel R Karlin, MD, MA Chief Medical Officer
Positive 12-Week Topline Results from Phase 2b Study in GAD: Strong 1 Durability of Effect after Single Dose of MM120 2 • Met the primary and all secondary endpoints with statistical significance • MCP-Mod analysis results support dose-response relationship for MM120 in GAD 3,4 • Large observed effect size of d=0.81 at 12 weeks is more than double the standard of care o Durability of at least 3 months after a single dose of MM120 observed • Statistically and clinically significant 21.9-point improvement in HAM-A score at week 12 (p=0.0025) 3 represents further improvement from four-week topline data o Rapid and durable clinical activity with continued improvement at week 12 5 o 48% clinical remission rate through 12-week observation period 2 o Clinically and statistically significant improvements on all analyzed secondary endpoints at week 12 • MM120 was well-tolerated with no related serious adverse events o Mostly transient, mild-to-moderate adverse events consistent with drug class and prior studies 6 o No drug-related serious adverse event (SAE) and no suicide-related safety signal • Supports long-term durability of single administration MM120 and we believe further supports advancement of 100 µg MM120 into Phase 3 development for GAD 1. Source: Study MMED008 internal study documents and calculations. Individual group results reported on 100 µg dose group vs. placebo. 2. Represents all analyzed secondary endpoints in week 12 topline analysis, including HAM-A, CGI-S and MADRS. 3. Based on 100 µg dose group; HAM-A scores based on ANCOVA LS Mean. Effect size based on post hoc calculation by study statistician using LS Mean change between group and pooled standard deviation of ending HAM-A scores across groups. 4. Examination of baseline group assignment for all of the studies (20 studies utilizing the HAM-A (Hamilton Anxiety Scale) and 1 study using the PARS (Pediatric Anxiety Scale) for the primary outcome measurement. Source: RB Hidalgo, J 13 Investor Presentation | March 2024 Psychopharmacol. 2007 Nov;21(8):864-72. 5. Remission defined as HAM-A score of ≤7. 6. Suicidality assessment based on reported adverse events.
Phase 2b Trial of MM120 Utilized Standard GAD Design and Endpoints 1 and was Aligned with FDA Draft Guidance for Drug Class • Standard GAD study design with endpoints that have supported registration for approved drugs • Randomized, double-blind, placebo-controlled, 12-week trial o Single administration of MM-120 or placebo o No psychotherapeutic intervention 2 o Trial design closely aligned with subsequently issued FDA 2023 Draft Guidance o Patients washed out of anxiety pharmacotherapy prior to randomization • Enrolled 198 patients with GAD • Five-arm dose optimization design with 1:1:1:1:1 randomization • Primary endpoint: change in Hamilton Anxiety Scale (HAM-A) at week 4 o Assessed by central rater blinded to treatment assignment and visit number 1. Source: Study MMED008 internal study documents and calculations. 14 Investor Presentation | March 2024 2. FDA 2023 Draft Guidance: Psychedelic Drugs: Considerations for Clinical Investigations.
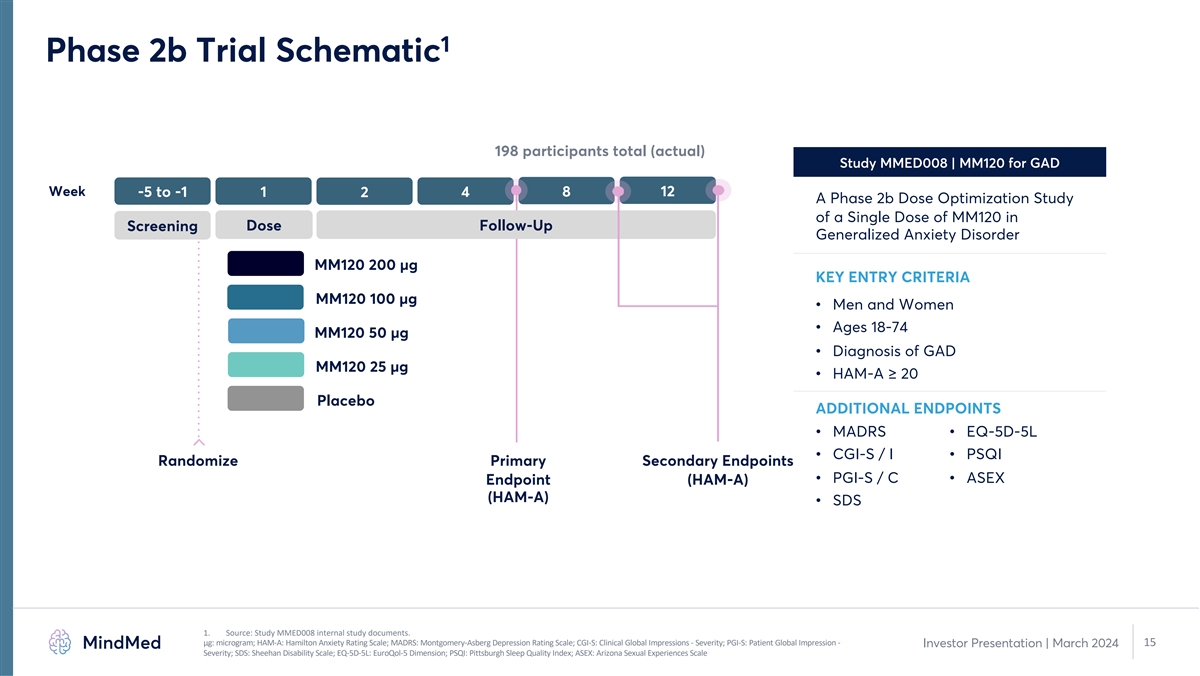
1 Phase 2b Trial Schematic 198 participants total (actual) Study MMED008 | MM120 for GAD Week -5 to -1 1 4 8 12 2 A Phase 2b Dose Optimization Study of a Single Dose of MM120 in Dose Follow-Up Screening Generalized Anxiety Disorder MM120 200 µg KEY ENTRY CRITERIA MM120 100 µg • Men and Women • Ages 18-74 MM120 50 µg • Diagnosis of GAD MM120 25 µg • HAM-A ≥ 20 Placebo ADDITIONAL ENDPOINTS • MADRS • EQ-5D-5L • CGI-S / I • PSQI Randomize Primary Secondary Endpoints • PGI-S / C • ASEX Endpoint (HAM-A) (HAM-A) • SDS 1. Source: Study MMED008 internal study documents. μg: microgram; HAM-A: Hamilton Anxiety Rating Scale; MADRS: Montgomery-Asberg Depression Rating Scale; CGI-S: Clinical Global Impressions - Severity; PGI-S: Patient Global Impression - 15 Investor Presentation | March 2024 Severity; SDS: Sheehan Disability Scale; EQ-5D-5L: EuroQol-5 Dimension; PSQI: Pittsburgh Sleep Quality Index; ASEX: Arizona Sexual Experiences Scale
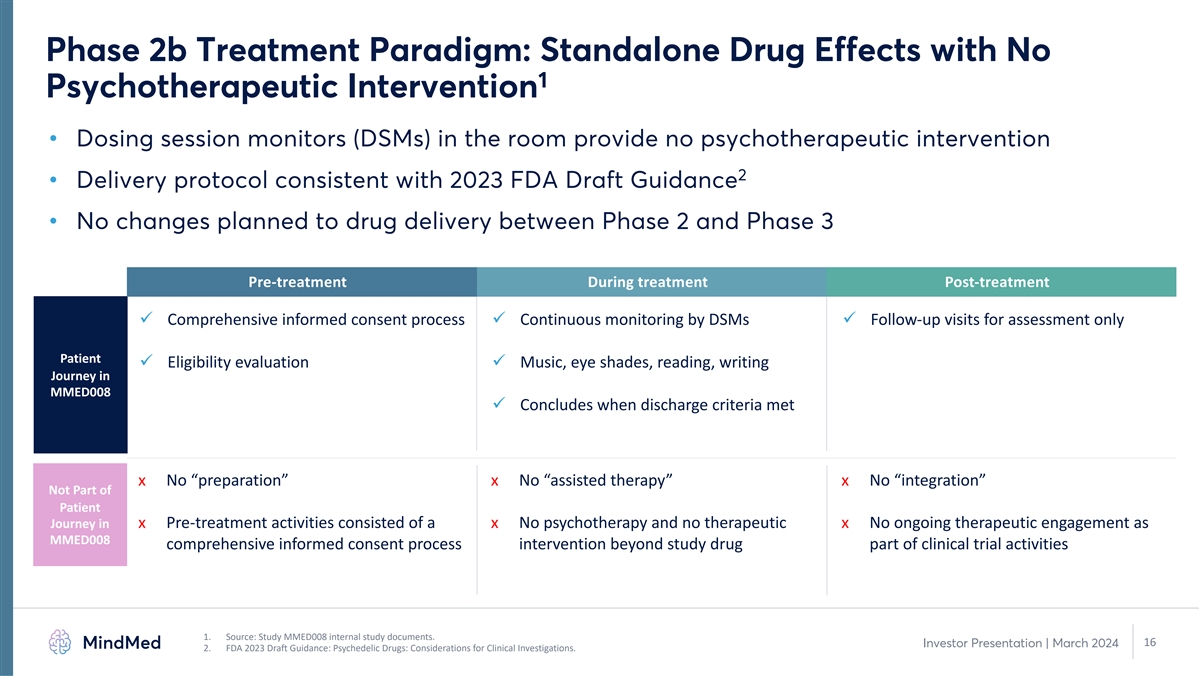
Phase 2b Treatment Paradigm: Standalone Drug Effects with No 1 Psychotherapeutic Intervention • Dosing session monitors (DSMs) in the room provide no psychotherapeutic intervention 2 • Delivery protocol consistent with 2023 FDA Draft Guidance • No changes planned to drug delivery between Phase 2 and Phase 3 Pre-treatment During treatment Post-treatment ü Comprehensive informed consent process ü Continuous monitoring by DSMs ü Follow-up visits for assessment only Patient ü Eligibility evaluation ü Music, eye shades, reading, writing Journey in MMED008 ü Concludes when discharge criteria met x No “preparation” x No “assisted therapy” x No “integration” Not Part of Patient Journey in x Pre-treatment activities consisted of a x No psychotherapy and no therapeutic x No ongoing therapeutic engagement as MMED008 comprehensive informed consent process intervention beyond study drug part of clinical trial activities 1. Source: Study MMED008 internal study documents. 16 Investor Presentation | March 2024 2. FDA 2023 Draft Guidance: Psychedelic Drugs: Considerations for Clinical Investigations.
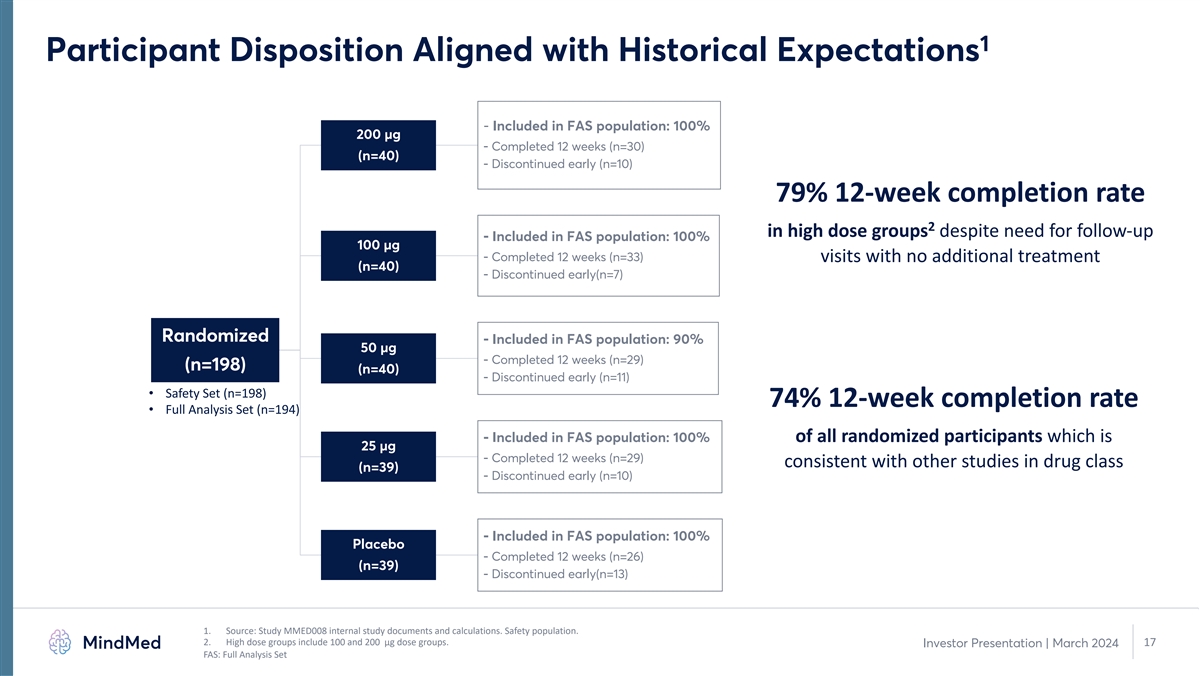
1 Participant Disposition Aligned with Historical Expectations - Included in FAS population: 100% 200 µg - Completed 12 weeks (n=30) (n=40) - Discontinued early (n=10) 79% 12-week completion rate 2 in high dose groups despite need for follow-up - Included in FAS population: 100% 100 µg - Completed 12 weeks (n=33) visits with no additional treatment (n=40) - Discontinued early(n=7) Randomized - Included in FAS population: 90% 50 µg - Completed 12 weeks (n=29) (n=198) (n=40) - Discontinued early (n=11) • Safety Set (n=198) 74% 12-week completion rate • Full Analysis Set (n=194) - Included in FAS population: 100% of all randomized participants which is 25 µg - Completed 12 weeks (n=29) consistent with other studies in drug class (n=39) - Discontinued early (n=10) - Included in FAS population: 100% Placebo - Completed 12 weeks (n=26) (n=39) - Discontinued early(n=13) 1. Source: Study MMED008 internal study documents and calculations. Safety population. 2. High dose groups include 100 and 200 µg dose groups. 17 Investor Presentation | March 2024 FAS: Full Analysis Set
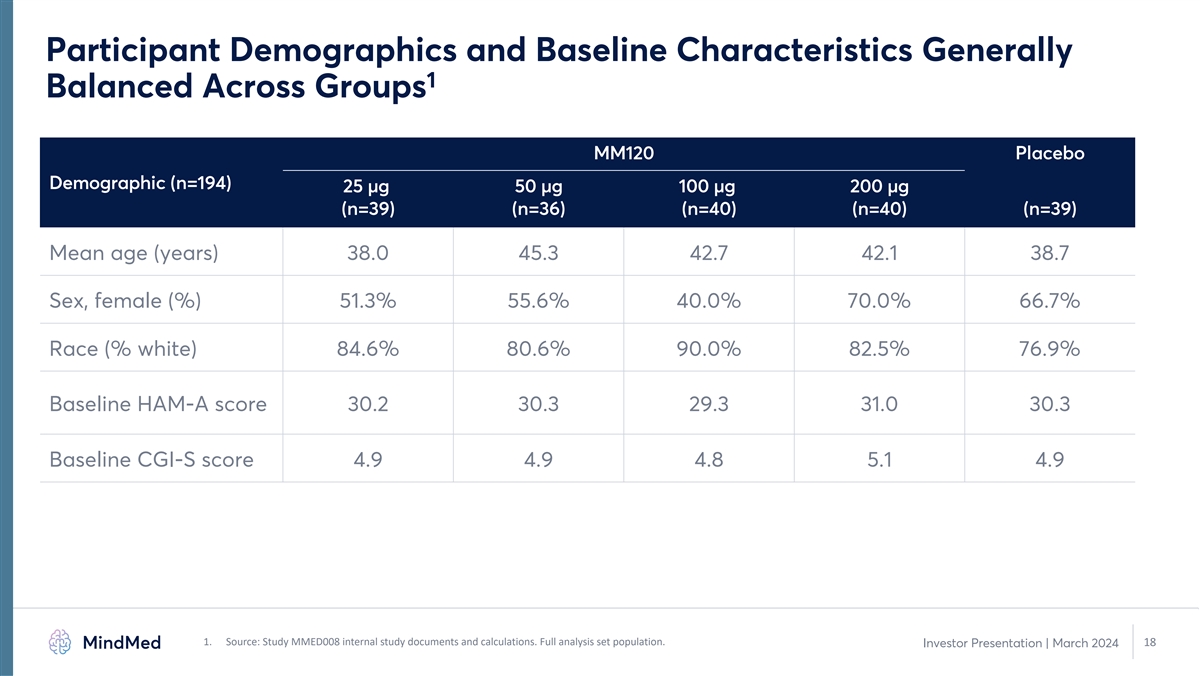
Participant Demographics and Baseline Characteristics Generally 1 Balanced Across Groups MM120 Placebo Demographic (n=194) 25 µg 50 µg 100 µg 200 µg (n=39) (n=36) (n=40) (n=40) (n=39) Mean age (years) 38.0 45.3 42.7 42.1 38.7 Sex, female (%) 51.3% 55.6% 40.0% 70.0% 66.7% Race (% white) 84.6% 80.6% 90.0% 82.5% 76.9% Baseline HAM-A score 30.2 30.3 29.3 31.0 30.3 Baseline CGI-S score 4.9 4.9 4.8 5.1 4.9 1. Source: Study MMED008 internal study documents and calculations. Full analysis set population. 18 Investor Presentation | March 2024
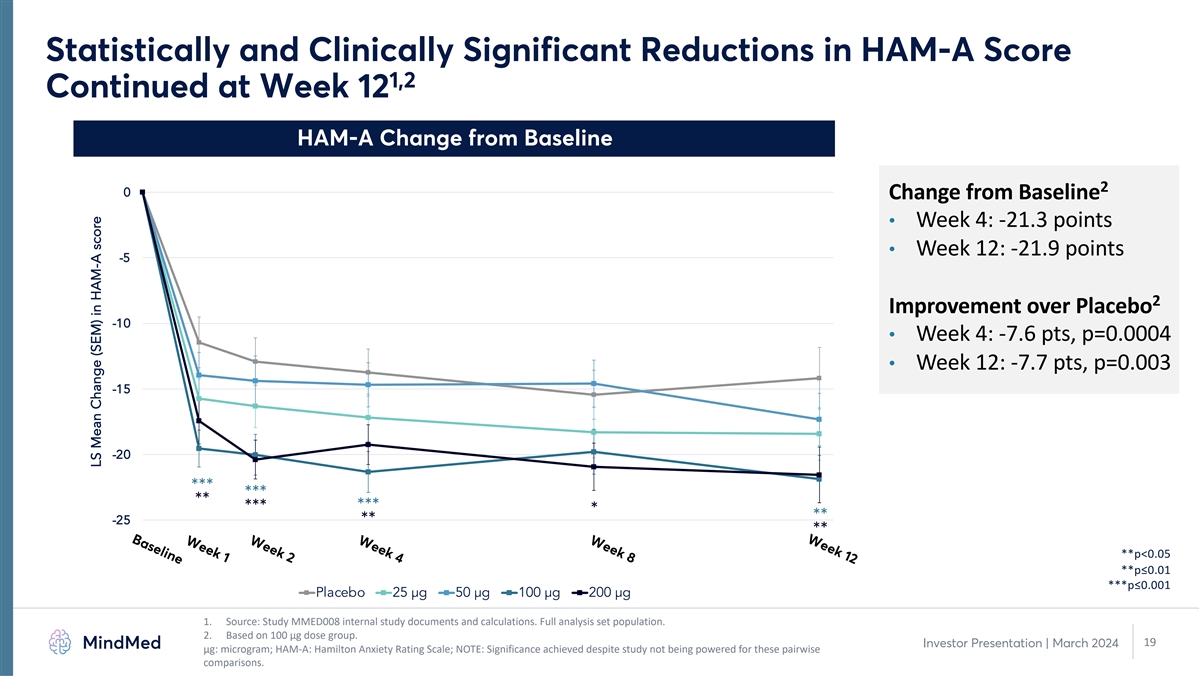
Week 12 Week 8 Week 4 Week 2 Week 1 Baseline Statistically and Clinically Significant Reductions in HAM-A Score 1,2 Continued at Week 12 HAM-A Change from Baseline 2 0 Change from Baseline • Week 4: -21.3 points • Week 12: -21.9 points -5 2 Improvement over Placebo -10 • Week 4: -7.6 pts, p=0.0004 • Week 12: -7.7 pts, p=0.003 -15 -20 *** *** ** *** *** * ** -25 ** ** **p<0.05 **p≤0.01 ***p≤0.001 Placebo 25 µg 50 µg 100 µg 200 µg 1. Source: Study MMED008 internal study documents and calculations. Full analysis set population. 2. Based on 100 µg dose group. 19 Investor Presentation | March 2024 μg: microgram; HAM-A: Hamilton Anxiety Rating Scale; NOTE: Significance achieved despite study not being powered for these pairwise comparisons. LS Mean Change (SEM) in HAM-A score
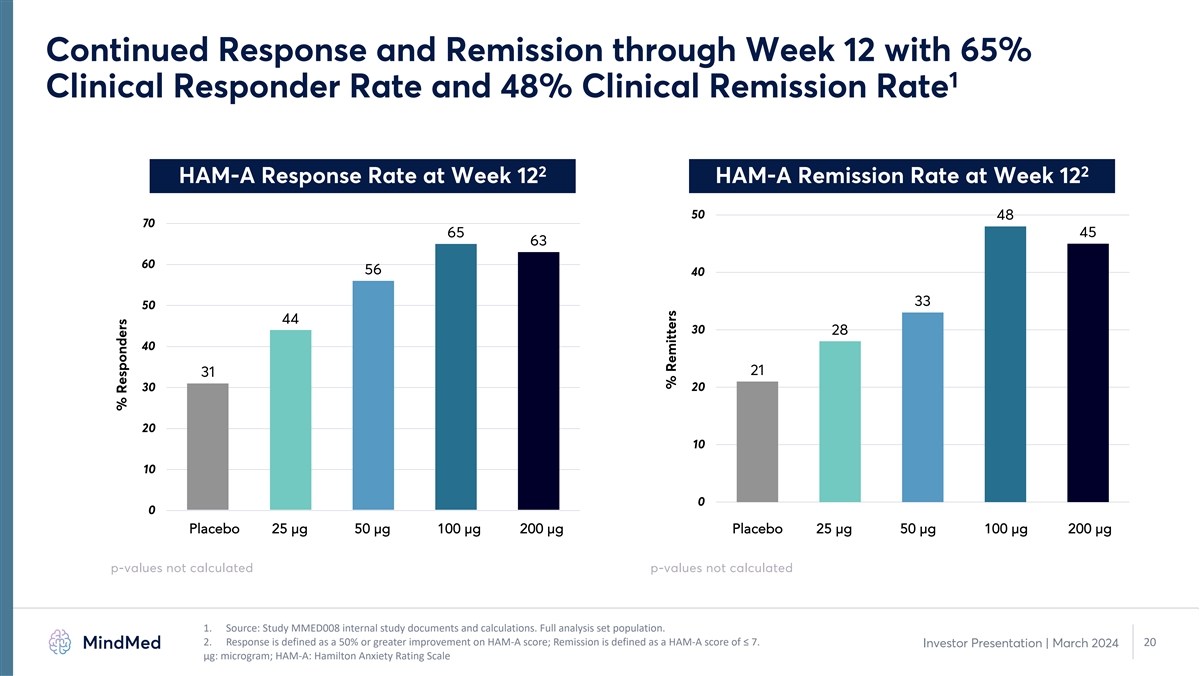
Continued Response and Remission through Week 12 with 65% 1 Clinical Responder Rate and 48% Clinical Remission Rate 2 2 HAM-A Response Rate at Week 12 HAM-A Remission Rate at Week 12 50 48 70 65 45 63 60 56 40 33 50 44 30 28 40 21 31 20 30 20 10 10 0 0 Placebo 25 µg 50 µg 100 µg 200 µg Placebo 25 µg 50 µg 100 µg 200 µg p-values not calculated p-values not calculated 1. Source: Study MMED008 internal study documents and calculations. Full analysis set population. 2. Response is defined as a 50% or greater improvement on HAM-A score; Remission is defined as a HAM-A score of ≤ 7. 20 Investor Presentation | March 2024 μg: microgram; HAM-A: Hamilton Anxiety Rating Scale % Responders % Remitters
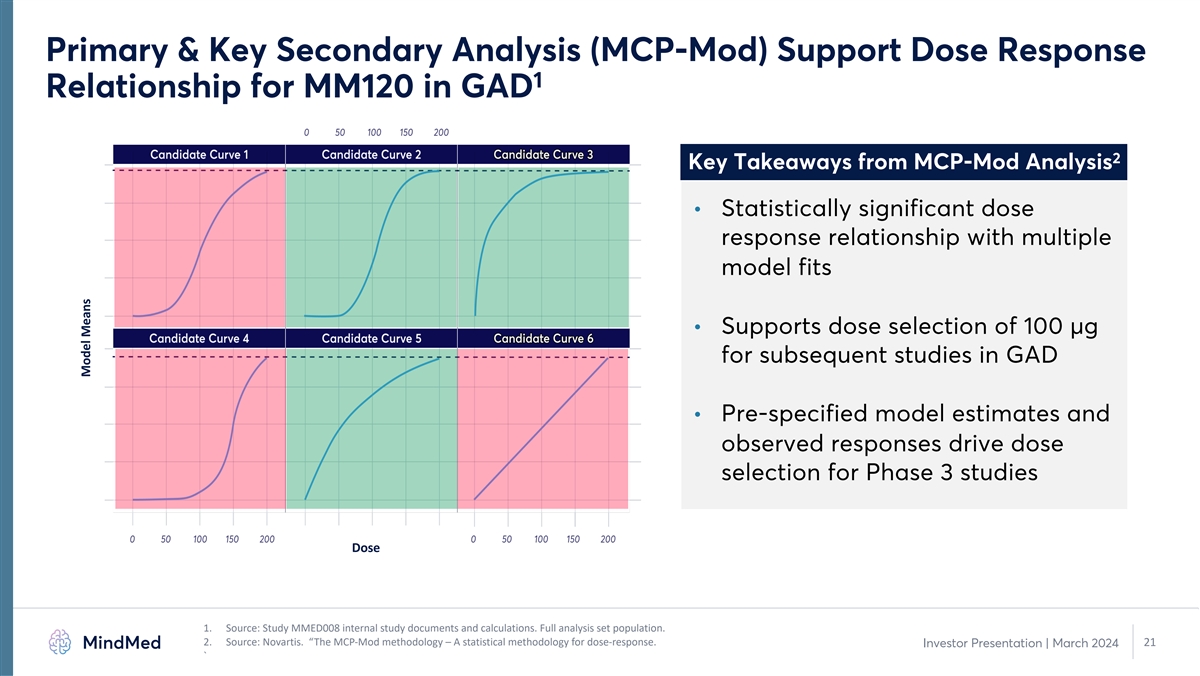
Primary & Key Secondary Analysis (MCP-Mod) Support Dose Response 1 Relationship for MM120 in GAD 2 Key Takeaways from MCP-Mod Analysis • Statistically significant dose response relationship with multiple model fits • Supports dose selection of 100 µg for subsequent studies in GAD • Pre-specified model estimates and observed responses drive dose selection for Phase 3 studies Dose 1. Source: Study MMED008 internal study documents and calculations. Full analysis set population. 2. Source: Novartis. “The MCP-Mod methodology – A statistical methodology for dose-response. 21 Investor Presentation | March 2024 ` Model Means
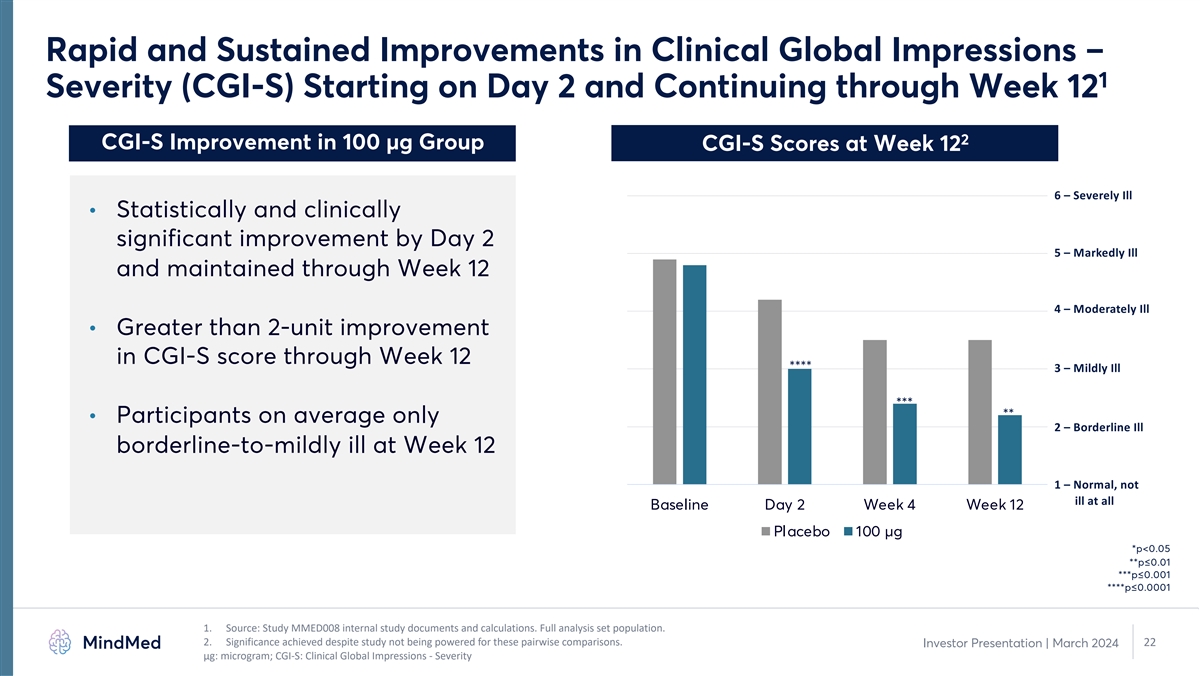
Rapid and Sustained Improvements in Clinical Global Impressions – 1 Severity (CGI-S) Starting on Day 2 and Continuing through Week 12 2 CGI-S Improvement in 100 µg Group CGI-S Scores at Week 12 6 – Severely Ill • Statistically and clinically significant improvement by Day 2 5 – Markedly Ill and maintained through Week 12 4 – Moderately Ill • Greater than 2-unit improvement in CGI-S score through Week 12 **** 3 – Mildly Ill *** ** • Participants on average only 2 – Borderline Ill borderline-to-mildly ill at Week 12 1 – Normal, not ill at all Baseline Day 2 Week 4 Week 12 Placebo 100 µg *p<0.05 **p≤0.01 ***p≤0.001 ****p≤0.0001 1. Source: Study MMED008 internal study documents and calculations. Full analysis set population. 2. Significance achieved despite study not being powered for these pairwise comparisons. 22 Investor Presentation | March 2024 μg: microgram; CGI-S: Clinical Global Impressions - Severity
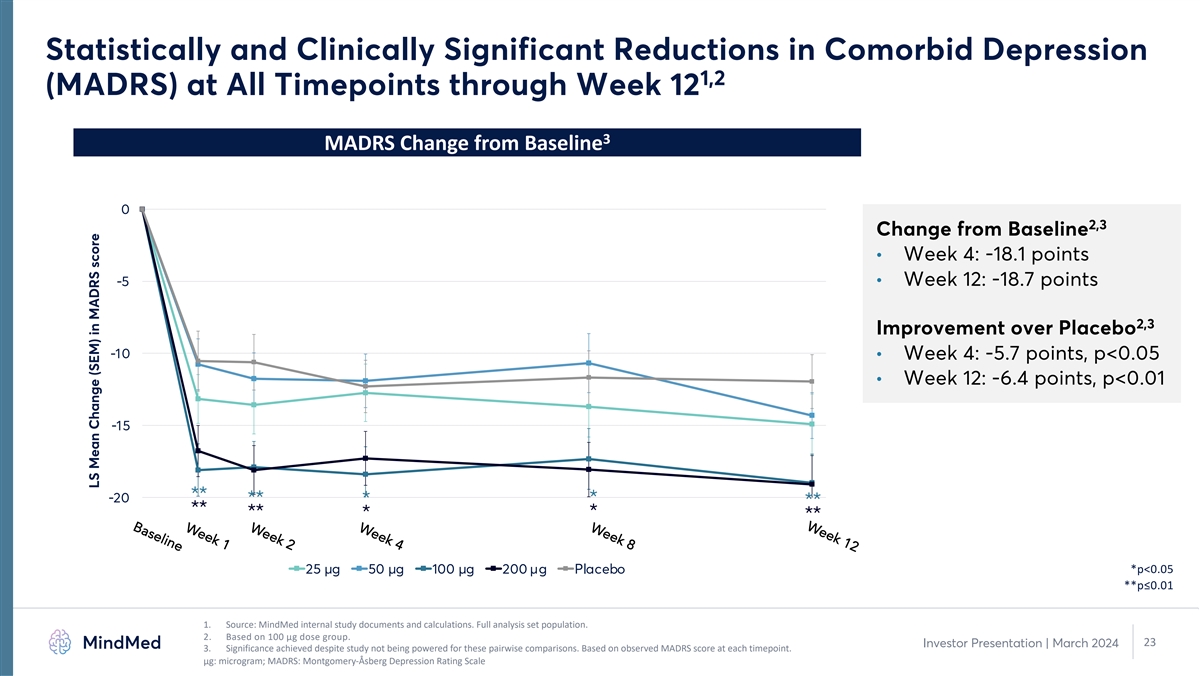
Week 12 Week 8 Week 4 Week 2 Week 1 Baseline Statistically and Clinically Significant Reductions in Comorbid Depression 1,2 (MADRS) at All Timepoints through Week 12 3 MADRS Change from Baseline 0 2,3 Change from Baseline • Week 4: -18.1 points • Week 12: -18.7 points -5 2,3 Improvement over Placebo -10 • Week 4: -5.7 points, p<0.05 • Week 12: -6.4 points, p<0.01 -15 ** -20 ** * * ** ** ** * * ** 25 µg 50 µg 100 µg 200 µg Placebo *p<0.05 **p≤0.01 1. Source: MindMed internal study documents and calculations. Full analysis set population. 2. Based on 100 µg dose group. 23 Investor Presentation | March 2024 3. Significance achieved despite study not being powered for these pairwise comparisons. Based on observed MADRS score at each timepoint. μg: microgram; MADRS: Montgomery-Åsberg Depression Rating Scale LS Mean Change (SEM) in MADRS score
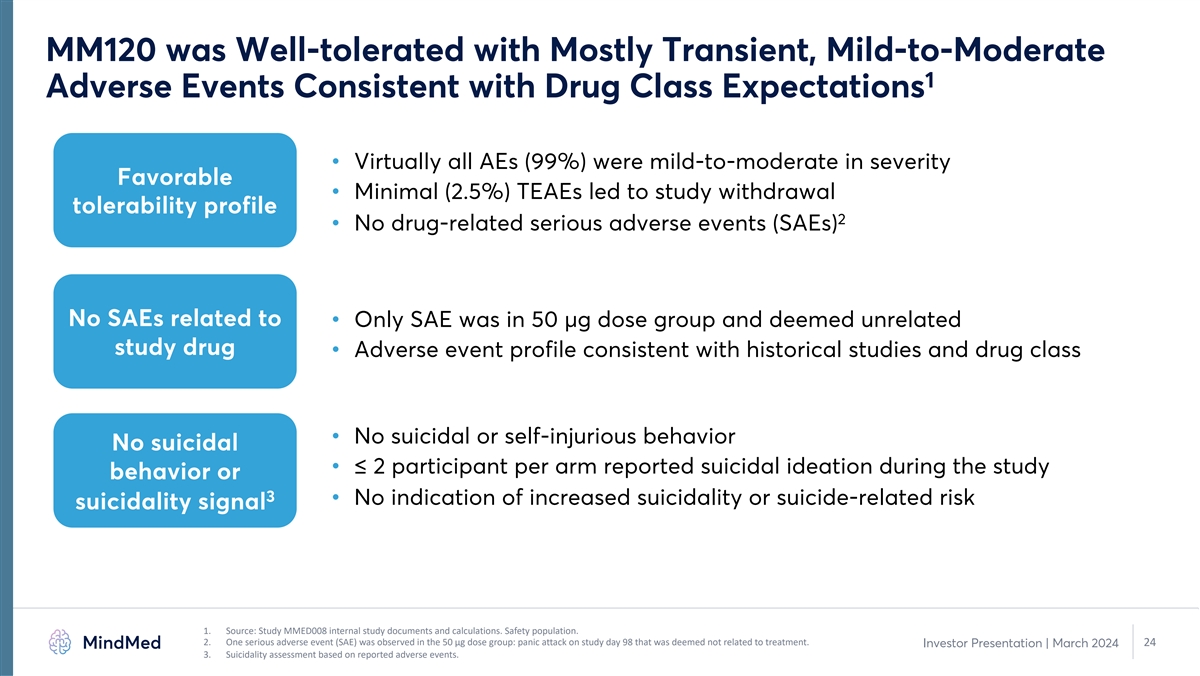
MM120 was Well-tolerated with Mostly Transient, Mild-to-Moderate 1 Adverse Events Consistent with Drug Class Expectations • Virtually all AEs (99%) were mild-to-moderate in severity Favorable • Minimal (2.5%) TEAEs led to study withdrawal tolerability profile 2 • No drug-related serious adverse events (SAEs) No SAEs related to • Only SAE was in 50 µg dose group and deemed unrelated study drug • Adverse event profile consistent with historical studies and drug class • No suicidal or self-injurious behavior No suicidal • ≤ 2 participant per arm reported suicidal ideation during the study behavior or 3 • No indication of increased suicidality or suicide-related risk suicidality signal 1. Source: Study MMED008 internal study documents and calculations. Safety population. 2. One serious adverse event (SAE) was observed in the 50 µg dose group: panic attack on study day 98 that was deemed not related to treatment. 24 Investor Presentation | March 2024 3. Suicidality assessment based on reported adverse events.
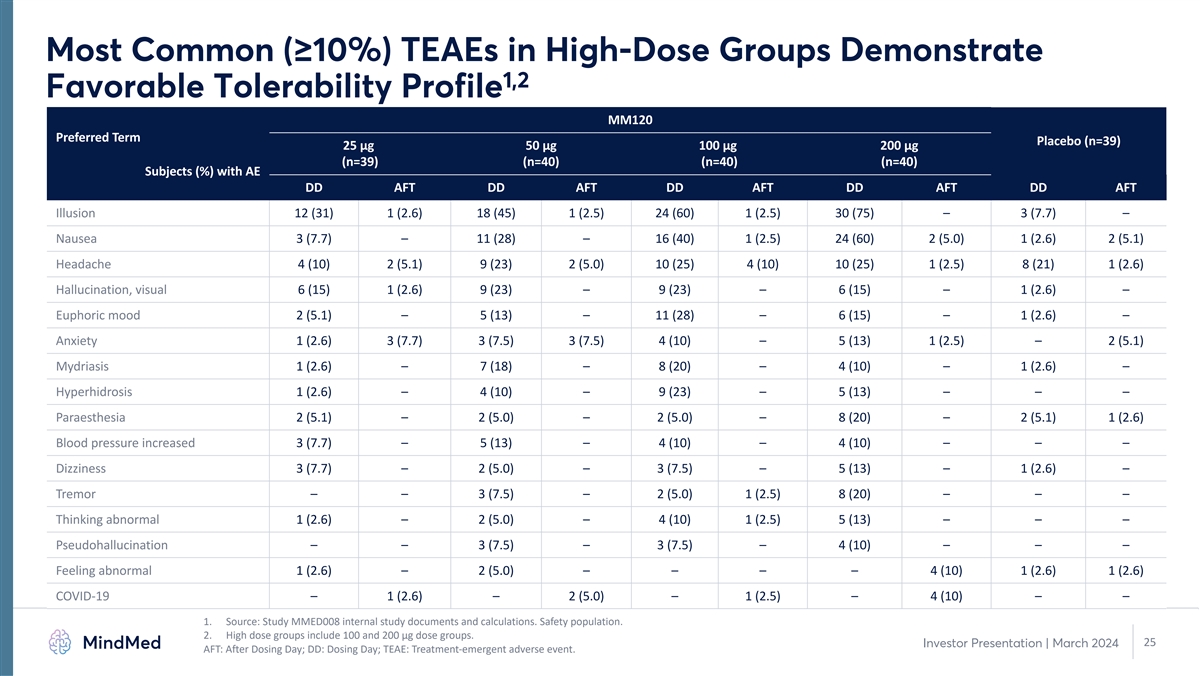
Most Common (≥10%) TEAEs in High-Dose Groups Demonstrate 1,2 Favorable Tolerability Profile MM120 Preferred Term Placebo (n=39) 25 µg 50 µg 100 µg 200 µg (n=39) (n=40) (n=40) (n=40) Subjects (%) with AE DD AFT DD AFT DD AFT DD AFT DD AFT Illusion 12 (31) 1 (2.6) 18 (45) 1 (2.5) 24 (60) 1 (2.5) 30 (75) – 3 (7.7) – Nausea 3 (7.7) – 11 (28) – 16 (40) 1 (2.5) 24 (60) 2 (5.0) 1 (2.6) 2 (5.1) Headache 4 (10) 2 (5.1) 9 (23) 2 (5.0) 10 (25) 4 (10) 10 (25) 1 (2.5) 8 (21) 1 (2.6) Hallucination, visual 6 (15) 1 (2.6) 9 (23) – 9 (23) – 6 (15) – 1 (2.6) – Euphoric mood 2 (5.1) – 5 (13) – 11 (28) – 6 (15) – 1 (2.6) – Anxiety 1 (2.6) 3 (7.7) 3 (7.5) 3 (7.5) 4 (10) – 5 (13) 1 (2.5) – 2 (5.1) Mydriasis 1 (2.6) – 7 (18) – 8 (20) – 4 (10) – 1 (2.6) – Hyperhidrosis 1 (2.6) – 4 (10) – 9 (23) – 5 (13) – – – Paraesthesia 2 (5.1) – 2 (5.0) – 2 (5.0) – 8 (20) – 2 (5.1) 1 (2.6) Blood pressure increased 3 (7.7) – 5 (13) – 4 (10) – 4 (10) – – – Dizziness 3 (7.7) – 2 (5.0) – 3 (7.5) – 5 (13) – 1 (2.6) – Tremor – – 3 (7.5) – 2 (5.0) 1 (2.5) 8 (20) – – – Thinking abnormal 1 (2.6) – 2 (5.0) – 4 (10) 1 (2.5) 5 (13) – – – Pseudohallucination – – 3 (7.5) – 3 (7.5) – 4 (10) – – – Feeling abnormal 1 (2.6) – 2 (5.0) – – – – 4 (10) 1 (2.6) 1 (2.6) COVID-19 – 1 (2.6) – 2 (5.0) – 1 (2.5) – 4 (10) – – 1. Source: Study MMED008 internal study documents and calculations. Safety population. 2. High dose groups include 100 and 200 µg dose groups. 25 Investor Presentation | March 2024 AFT: After Dosing Day; DD: Dosing Day; TEAE: Treatment-emergent adverse event.

MM120 LSD-D-tartrate for Generalized Anxiety Disorder (GAD) MM120 ODT PK Bridging Study Daniel R Karlin, MD, MA Chief Medical Officer
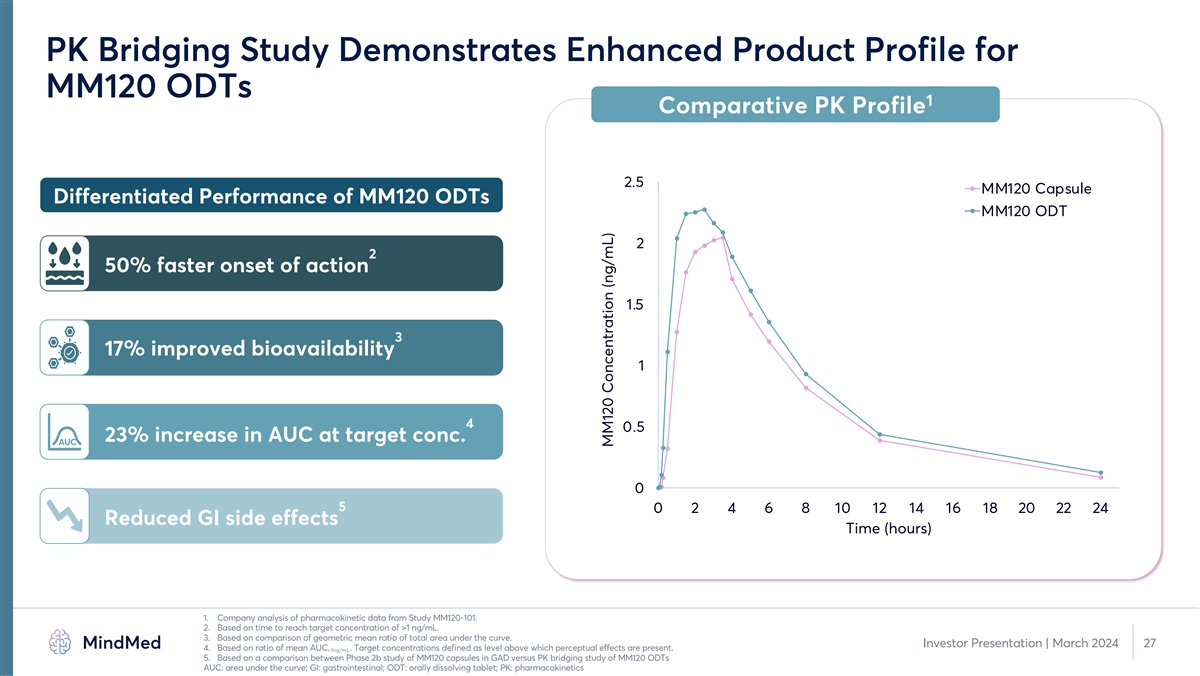
PK Bridging Study Demonstrates Enhanced Product Profile for MM120 ODTs 1 Comparative PK Profile 2.5 MM120 Capsule Differentiated Performance of MM120 ODTs MM120 ODT 2 2 50% faster onset of action 1.5 3 17% improved bioavailability 1 4 0.5 23% increase in AUC at target conc. AUC 0 5 0 2 4 6 8 10 12 14 16 18 20 22 24 Reduced GI side effects Time (hours) 1. Company analysis of pharmacokinetic data from Study MM120-101. 2. Based on time to reach target concentration of >1 ng/mL. 3. Based on comparison of geometric mean ratio of total area under the curve. Investor Presentation | March 2024 27 4. Based on ratio of mean AUC . Target concentrations defined as level above which perceptual effects are present. >1ng/mL 5. Based on a comparison between Phase 2b study of MM120 capsules in GAD versus PK bridging study of MM120 ODTs AUC: area under the curve; GI: gastrointestinal; ODT: orally dissolving tablet; PK: pharmacokinetics MM120 Concentration (ng/mL)
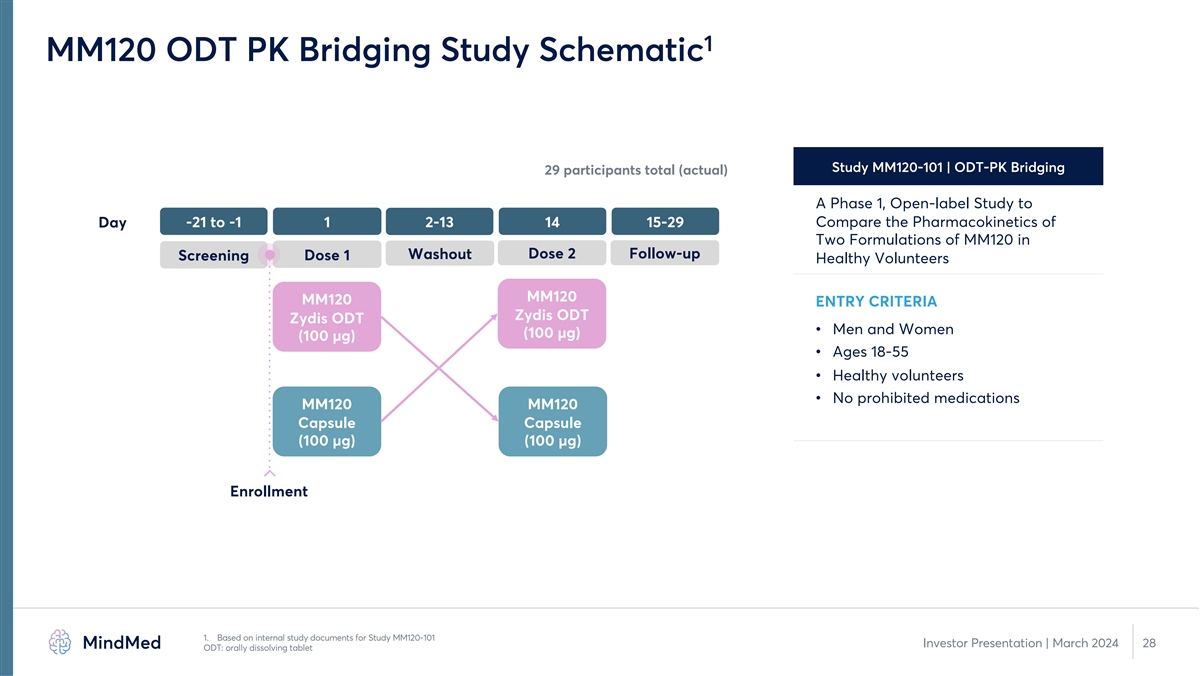
1 MM120 ODT PK Bridging Study Schematic Study MM120-101 | ODT-PK Bridging 29 participants total (actual) A Phase 1, Open-label Study to Compare the Pharmacokinetics of Day -21 to -1 1 2-13 14 15-29 Two Formulations of MM120 in Washout Dose 2 Follow-up Screening Dose 1 Healthy Volunteers MM120 MM120 ENTRY CRITERIA Zydis ODT Zydis ODT • Men and Women (100 µg) (100 µg) • Ages 18-55 • Healthy volunteers • No prohibited medications MM120 MM120 Capsule Capsule (100 µg) (100 µg) Enrollment 1. Based on internal study documents for Study MM120-101 Investor Presentation | March 2024 28 ODT: orally dissolving tablet
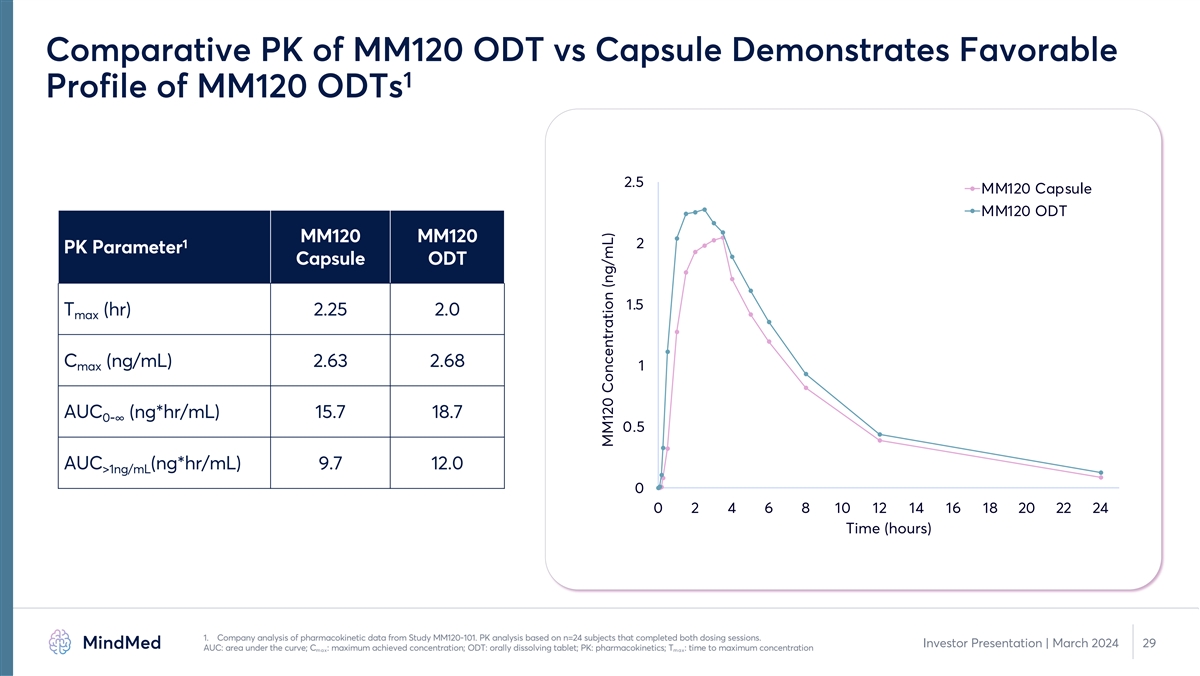
Comparative PK of MM120 ODT vs Capsule Demonstrates Favorable 1 Profile of MM120 ODTs 2.5 MM120 Capsule MM120 ODT MM120 MM120 1 2 PK Parameter Capsule ODT 1.5 T (hr) 2.25 2.0 max C (ng/mL) 2.63 2.68 max 1 AUC (ng*hr/mL) 15.7 18.7 0-∞ 0.5 AUC (ng*hr/mL) 9.7 12.0 >1ng/mL 0 0 2 4 6 8 10 12 14 16 18 20 22 24 Time (hours) 1. Company analysis of pharmacokinetic data from Study MM120-101. PK analysis based on n=24 subjects that completed both dosing sessions. Investor Presentation | March 2024 29 AUC: area under the curve; C : maximum achieved concentration; ODT: orally dissolving tablet; PK: pharmacokinetics; T : time to maximum concentration max max MM120 Concentration (ng/mL)
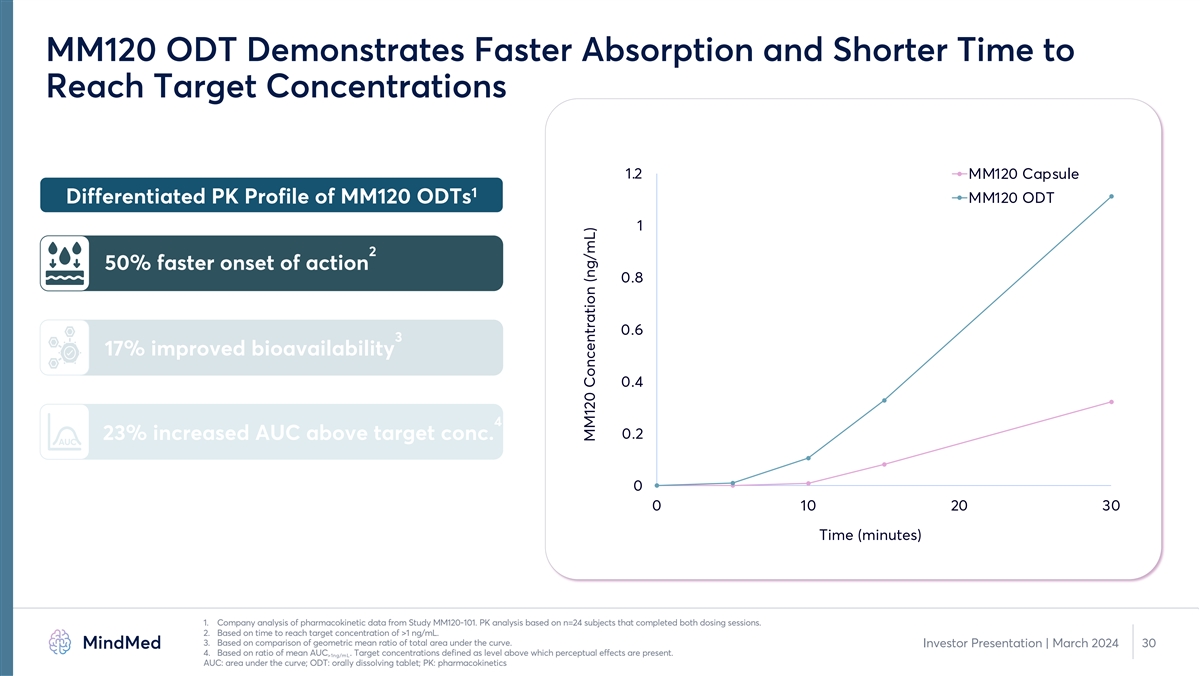
MM120 ODT Demonstrates Faster Absorption and Shorter Time to Reach Target Concentrations 1.2 MM120 Capsule 1 MM120 ODT Differentiated PK Profile of MM120 ODTs 1 2 50% faster onset of action 0.8 0.6 3 17% improved bioavailability 0.4 4 0.2 23% increased AUC above target conc. AUC 0 0 10 20 30 Time (minutes) 1. Company analysis of pharmacokinetic data from Study MM120-101. PK analysis based on n=24 subjects that completed both dosing sessions. 2. Based on time to reach target concentration of >1 ng/mL. 3. Based on comparison of geometric mean ratio of total area under the curve. Investor Presentation | March 2024 30 4. Based on ratio of mean AUC . Target concentrations defined as level above which perceptual effects are present. >1ng/mL AUC: area under the curve; ODT: orally dissolving tablet; PK: pharmacokinetics MM120 Concentration (ng/mL)
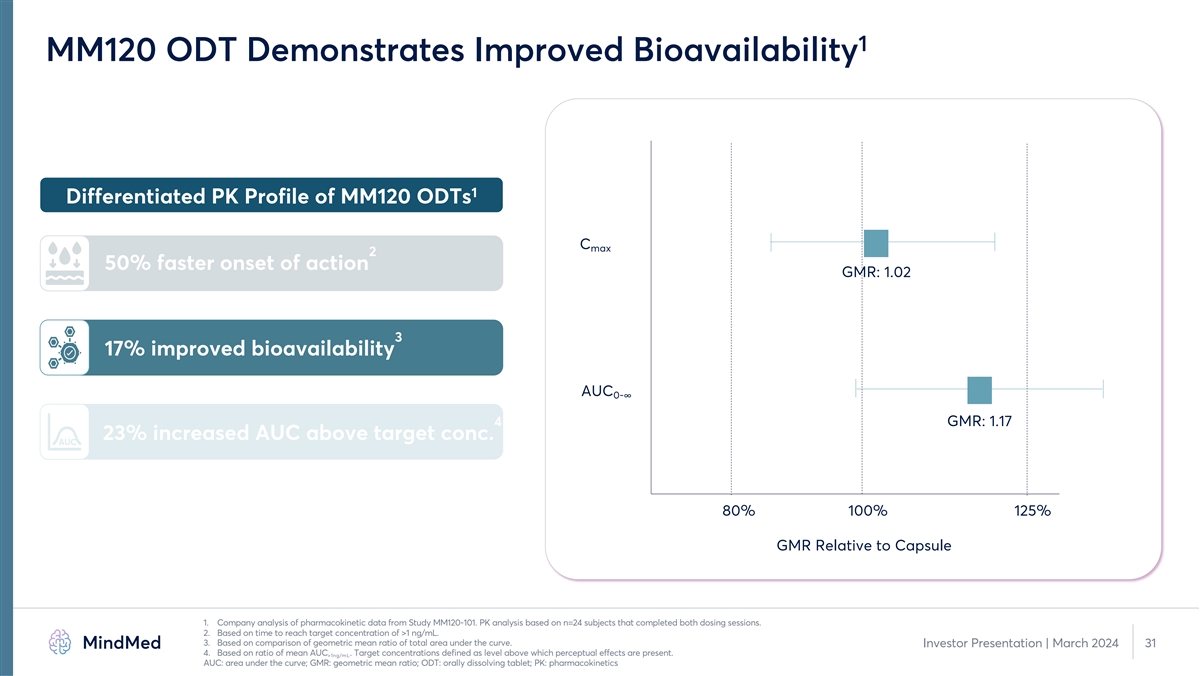
1 MM120 ODT Demonstrates Improved Bioavailability 1 Differentiated PK Profile of MM120 ODTs C max 2 50% faster onset of action GMR: 1.02 3 17% improved bioavailability AUC 0-∞ 4 GMR: 1.17 23% increased AUC above target conc. AUC 80% 100% 125% GMR Relative to Capsule 1. Company analysis of pharmacokinetic data from Study MM120-101. PK analysis based on n=24 subjects that completed both dosing sessions. 2. Based on time to reach target concentration of >1 ng/mL. 3. Based on comparison of geometric mean ratio of total area under the curve. Investor Presentation | March 2024 31 4. Based on ratio of mean AUC . Target concentrations defined as level above which perceptual effects are present. >1ng/mL AUC: area under the curve; GMR: geometric mean ratio; ODT: orally dissolving tablet; PK: pharmacokinetics
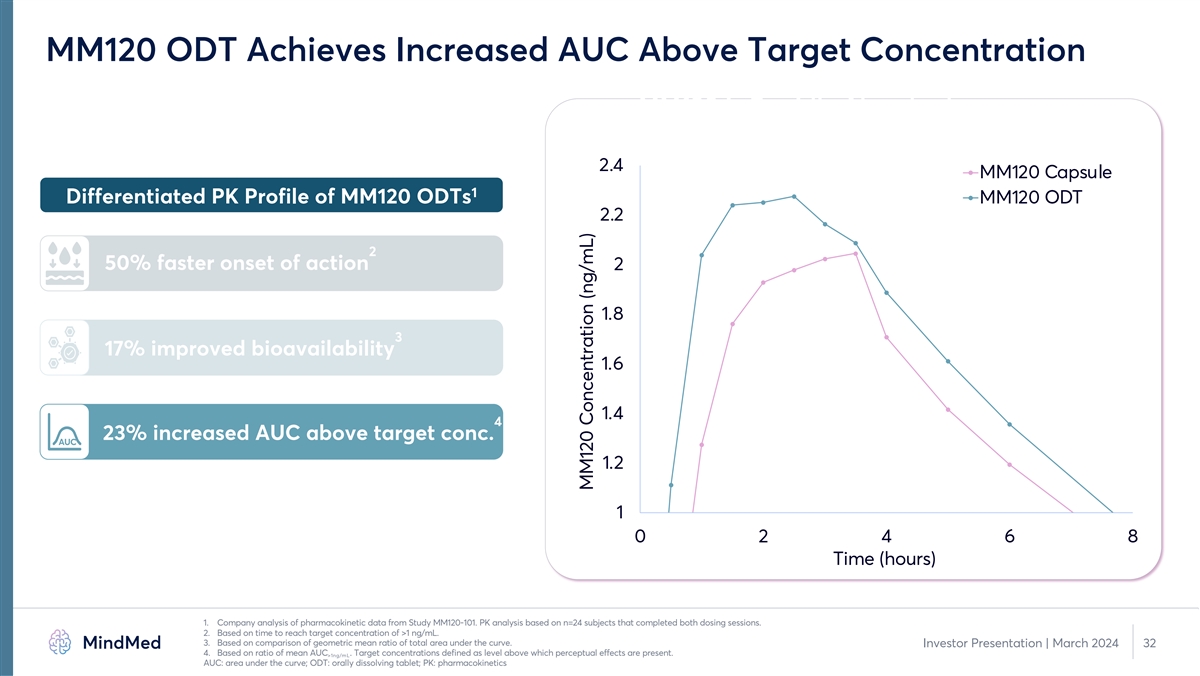
MM120 ODT Achieves Increased AUC Above Target Concentration MM120 is Rapidly Absorbed 2.4 MM120 Capsule 1 Differentiated PK Profile of MM120 ODTs MM120 ODT 2.2 2 50% faster onset of action 2 1.8 3 17% improved bioavailability 1.6 1.4 4 23% increased AUC above target conc. AUC 1.2 1 0 2 4 6 8 Time (hours) 1. Company analysis of pharmacokinetic data from Study MM120-101. PK analysis based on n=24 subjects that completed both dosing sessions. 2. Based on time to reach target concentration of >1 ng/mL. 3. Based on comparison of geometric mean ratio of total area under the curve. Investor Presentation | March 2024 32 4. Based on ratio of mean AUC . Target concentrations defined as level above which perceptual effects are present. >1ng/mL AUC: area under the curve; ODT: orally dissolving tablet; PK: pharmacokinetics MM120 Concentration (ng/mL)

Summary Comments for MM120 Development Plan Robert Barrow Chief Executive Officer
Multiple Studies Support Phase 3 Development of MM120 1 • Achieved goals of Phase 2 development o Characterized dose-response to inform dose selection in GAD o Large, statistically significant and clinically meaningful effect in GAD o Rapid and durable therapeutic benefits on validated endpoint o Standalone drug effect in absence of psychotherapeutic intervention • Multiple double-blind, placebo-controlled studies supporting activity of MM120 o Phase 2b randomized, placebo-controlled dose optimization trial in GAD (Study MMED008) o One prior modern, randomized, placebo-controlled IIT of lysergide in anxiety disorders o Over twenty legacy studies of lysergide in anxiety and other neurotic disorders • Phase 2b data supports dose selection and advancement into Phase 3 development 1. Source: Study MMED008 internal study documents and calculations. 34 Investor Presentation | March 2024
MM120 Development Pathway 1 • Two Phase 3 pivotal clinical trials in planning o 12-week randomized, placebo-controlled primary efficacy study design o Open-label extension to establish retreatment parameters o Expect to initiate Phase 3 development in the second half of 2024 • Key design elements expected to be consistent between Phase 2b and Phase 3 studies o Hamilton Anxiety Scale (HAM-A) at week 4 expected primary endpoint o Limited changes to key inclusion/exclusion criteria o No planned change in dosing session monitoring protocol 1. Phase 3 and subsequent clinical study design subject to regulatory discussion and review, including at potential End of Phase 2 meeting. 35 Investor Presentation | March 2024
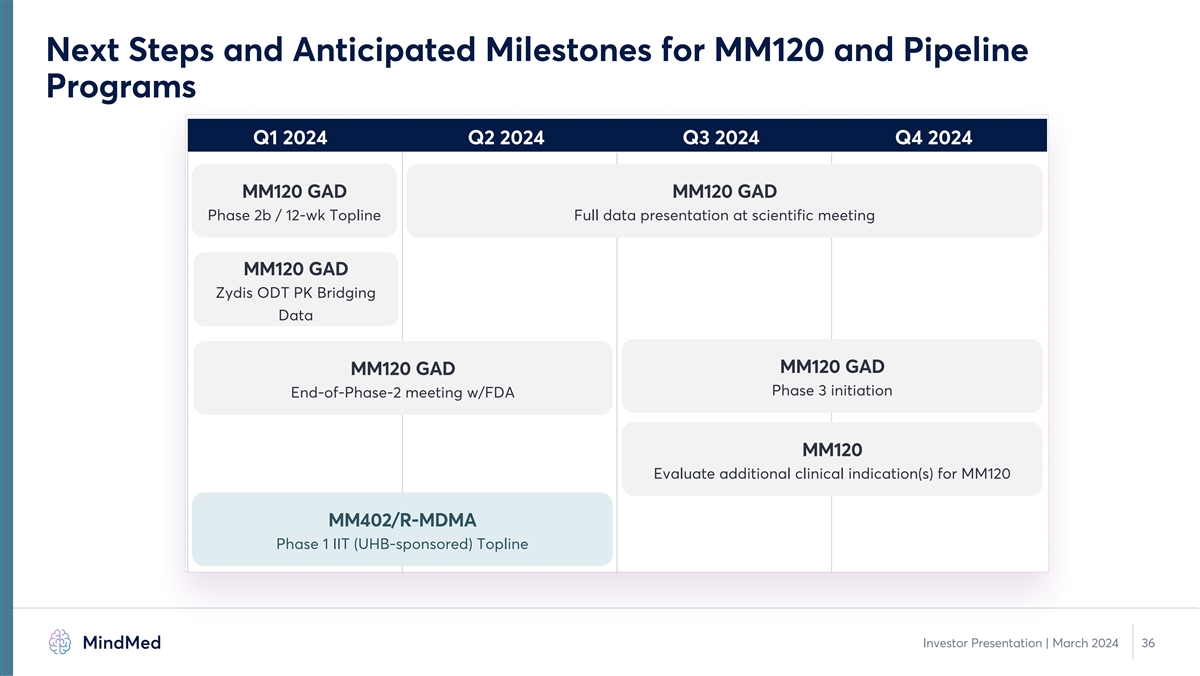
Next Steps and Anticipated Milestones for MM120 and Pipeline Programs Q1 2024 Q2 2024 Q3 2024 Q4 2024 MM120 GAD MM120 GAD Phase 2b / 12-wk Topline Full data presentation at scientific meeting MM120 GAD Zydis ODT PK Bridging Data MM120 GAD MM120 GAD Phase 3 initiation End-of-Phase-2 meeting w/FDA MM120 Evaluate additional clinical indication(s) for MM120 MM402/R-MDMA Phase 1 IIT (UHB-sponsored) Topline Investor Presentation | March 2024 36

Q&A 37 Investor Presentation | March 2024

Appendix
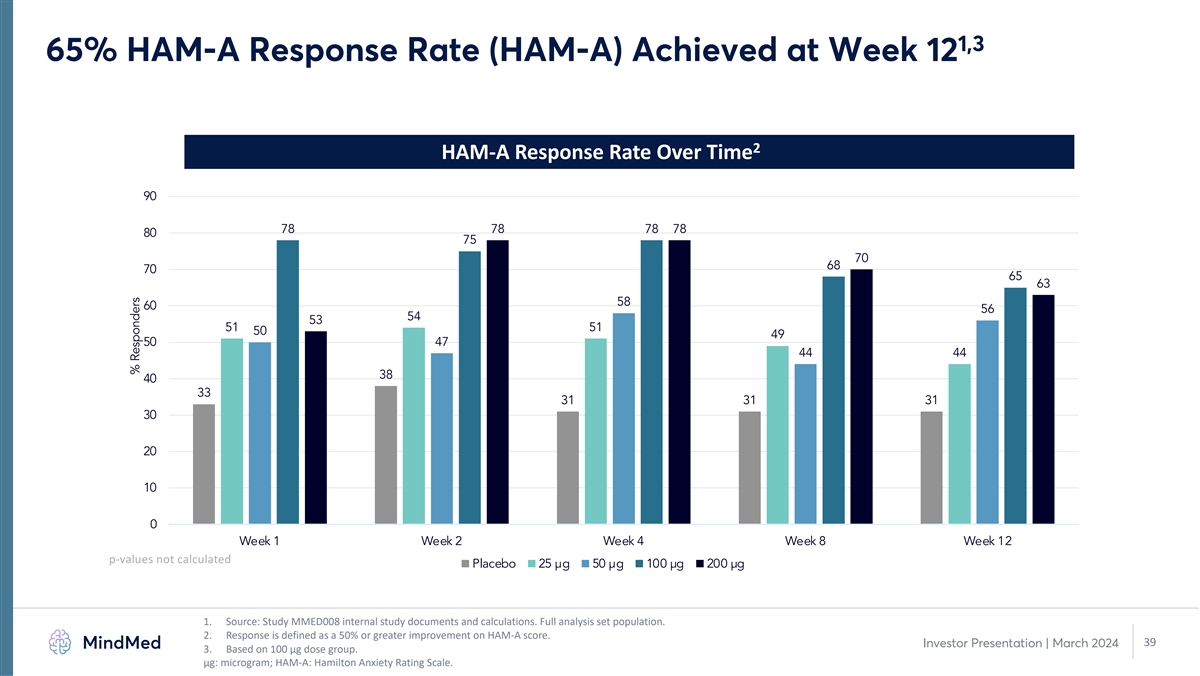
1,3 65% HAM-A Response Rate (HAM-A) Achieved at Week 12 2 HAM-A Response Rate Over Time 90 78 78 78 78 80 75 70 68 70 65 63 58 60 56 54 53 51 51 50 49 50 47 44 44 38 40 33 31 31 31 30 20 10 0 Week 1 Week 2 Week 4 Week 8 Week 12 p-values not calculated Placebo 25 µg 50 µg 100 µg 200 µg 1. Source: Study MMED008 internal study documents and calculations. Full analysis set population. 2. Response is defined as a 50% or greater improvement on HAM-A score. 39 Investor Presentation | March 2024 3. Based on 100 µg dose group. μg: microgram; HAM-A: Hamilton Anxiety Rating Scale. % Responders
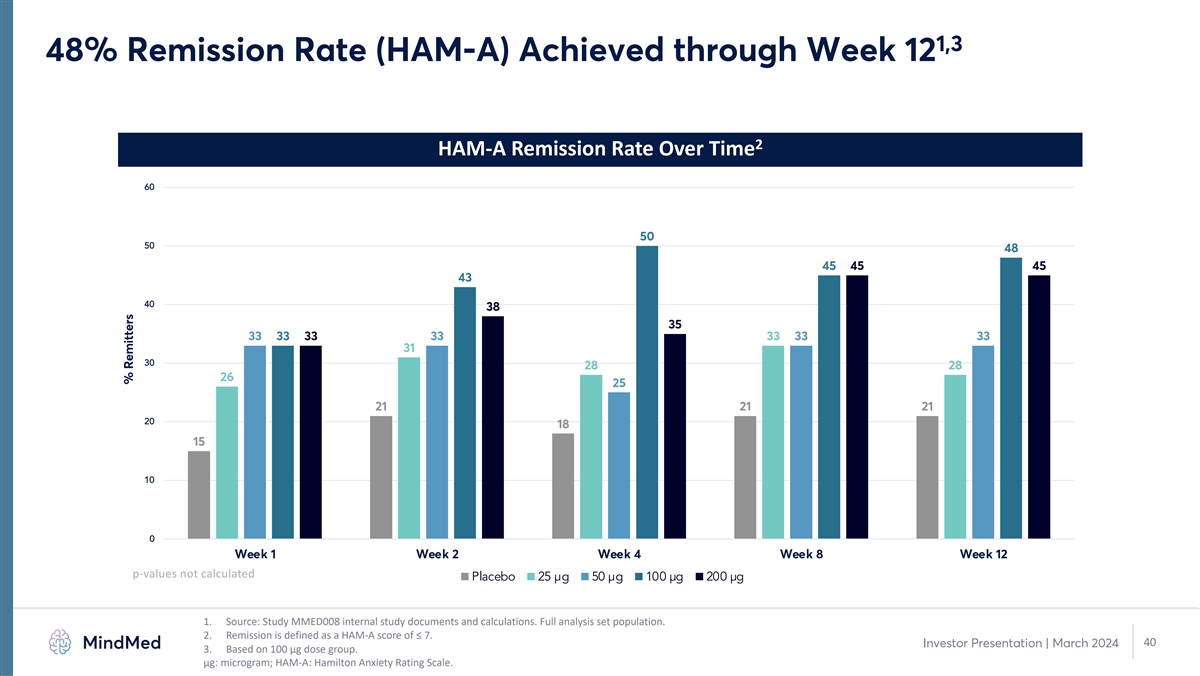
1,3 48% Remission Rate (HAM-A) Achieved through Week 12 2 HAM-A Remission Rate Over Time 60 50 50 48 45 45 45 43 40 38 35 33 33 33 33 33 33 33 31 30 28 28 26 25 21 21 21 20 18 15 10 0 Week 1 Week 2 Week 4 Week 8 Week 12 p-values not calculated Placebo 25 µg 50 µg 100 µg 200 µg 1. Source: Study MMED008 internal study documents and calculations. Full analysis set population. 2. Remission is defined as a HAM-A score of ≤ 7. 40 Investor Presentation | March 2024 3. Based on 100 µg dose group. μg: microgram; HAM-A: Hamilton Anxiety Rating Scale. % Remitters
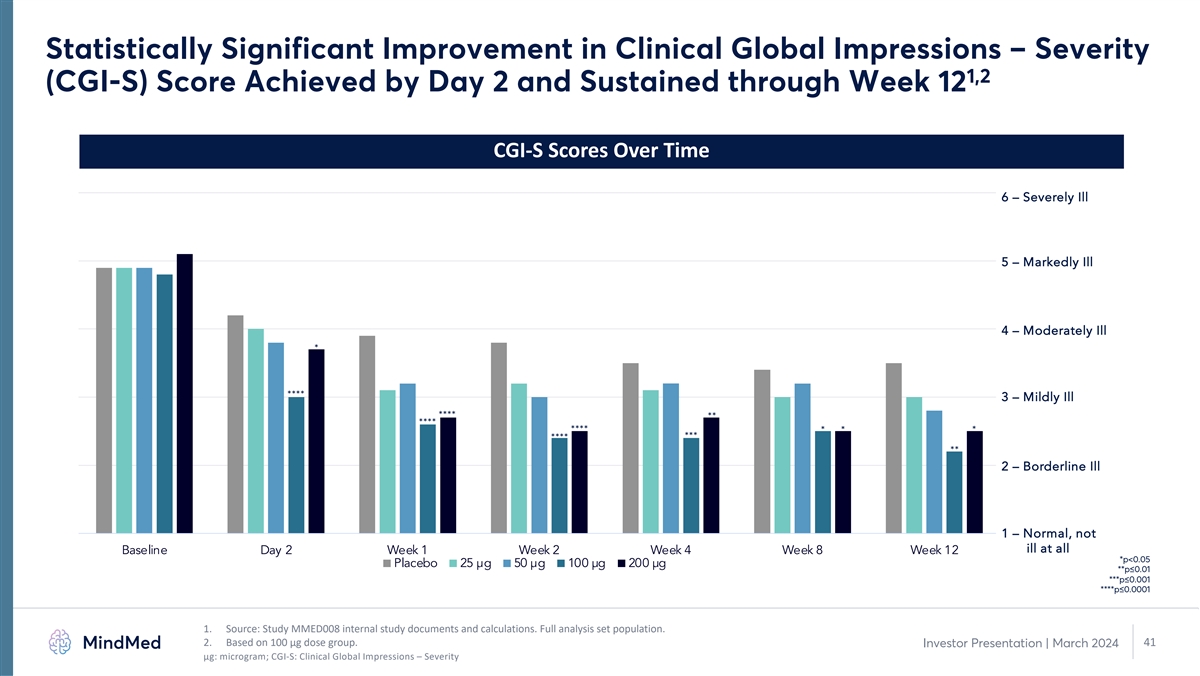
Statistically Significant Improvement in Clinical Global Impressions – Severity 1,2 (CGI-S) Score Achieved by Day 2 and Sustained through Week 12 CGI-S Scores Over Time 6 – Severely Ill 5 – Markedly Ill 4 – Moderately Ill * **** 3 – Mildly Ill **** ** **** **** * * * *** **** ** 2 – Borderline Ill 1 – Normal, not ill at all Baseline Day 2 Week 1 Week 2 Week 4 Week 8 Week 12 *p<0.05 Placebo 25 µg 50 µg 100 µg 200 µg **p≤0.01 ***p≤0.001 ****p≤0.0001 1. Source: Study MMED008 internal study documents and calculations. Full analysis set population. 2. Based on 100 µg dose group. 41 Investor Presentation | March 2024 μg: microgram; CGI-S: Clinical Global Impressions – Severity
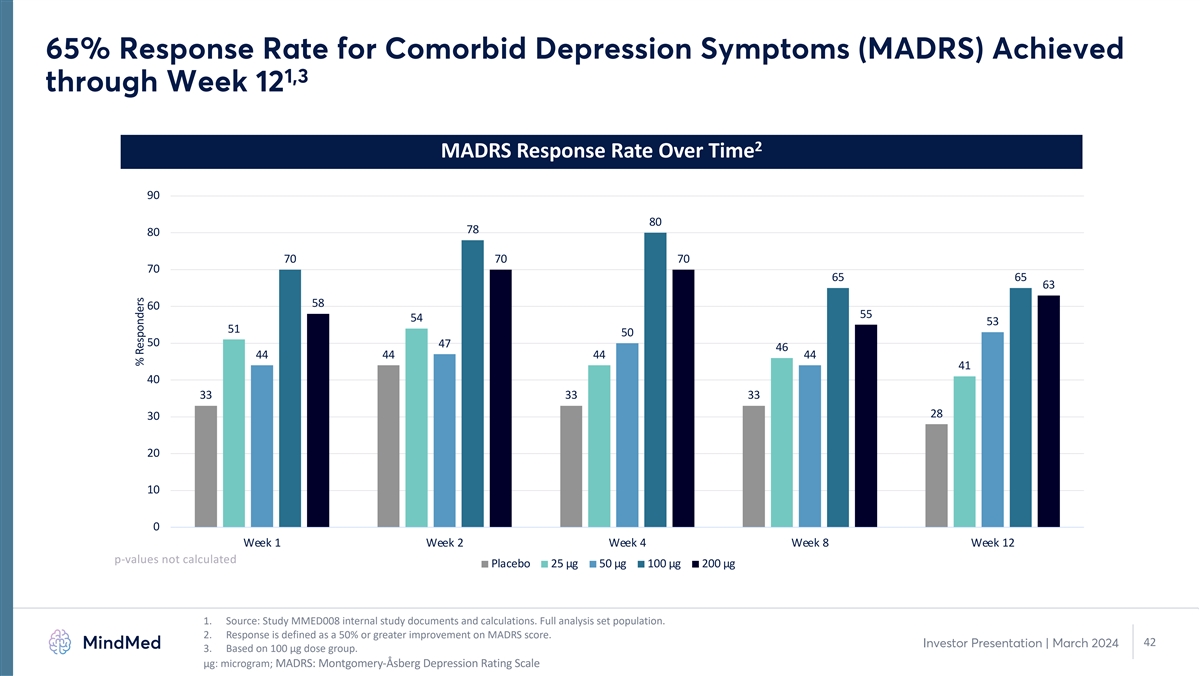
65% Response Rate for Comorbid Depression Symptoms (MADRS) Achieved 1,3 through Week 12 2 MADRS Response Rate Over Time 90 80 78 80 70 70 70 70 65 65 63 58 60 55 54 53 51 50 50 47 46 44 44 44 44 41 40 33 33 33 28 30 20 10 0 Week 1 Week 2 Week 4 Week 8 Week 12 p-values not calculated Placebo 25 µg 50 µg 100 µg 200 µg 1. Source: Study MMED008 internal study documents and calculations. Full analysis set population. 2. Response is defined as a 50% or greater improvement on MADRS score. 42 Investor Presentation | March 2024 3. Based on 100 µg dose group. μg: microgram; MADRS: Montgomery-Åsberg Depression Rating Scale % Responders
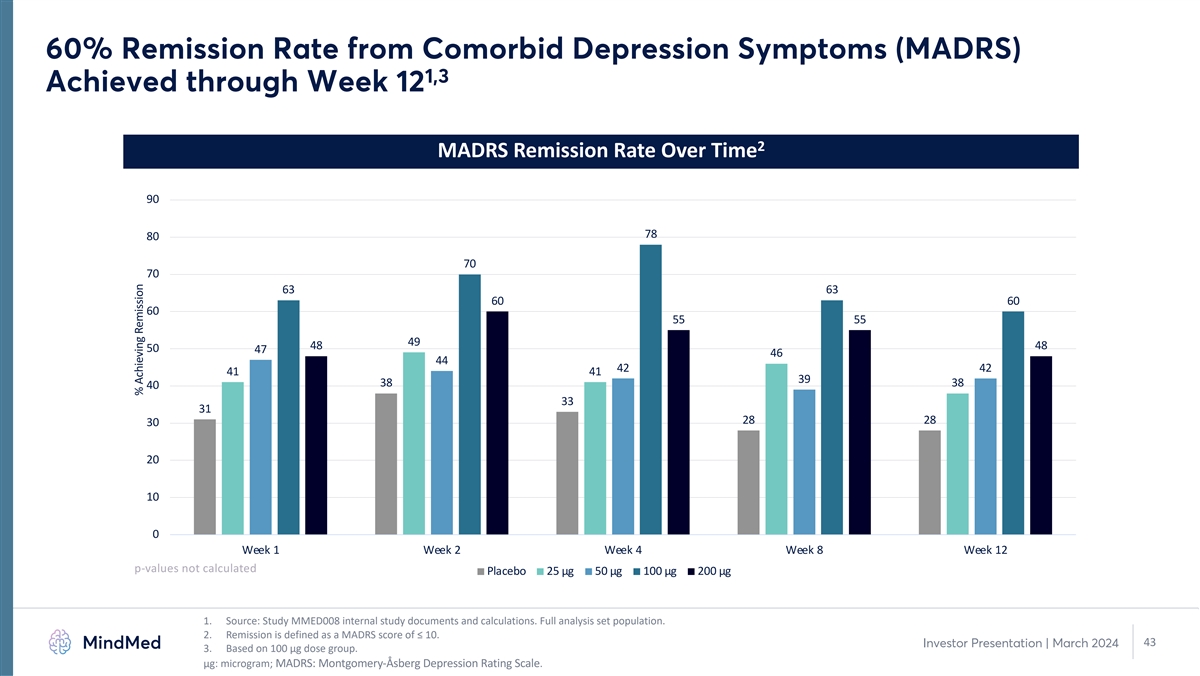
60% Remission Rate from Comorbid Depression Symptoms (MADRS) 1,3 Achieved through Week 12 2 MADRS Remission Rate Over Time 90 78 80 70 70 63 63 60 60 60 55 55 49 48 48 50 47 46 44 42 42 41 41 39 38 38 40 33 31 28 28 30 20 10 0 Week 1 Week 2 Week 4 Week 8 Week 12 p-values not calculated Placebo 25 µg 50 µg 100 µg 200 µg 1. Source: Study MMED008 internal study documents and calculations. Full analysis set population. 2. Remission is defined as a MADRS score of ≤ 10. 43 Investor Presentation | March 2024 3. Based on 100 µg dose group. μg: microgram; MADRS: Montgomery-Åsberg Depression Rating Scale. % Achieving Remission
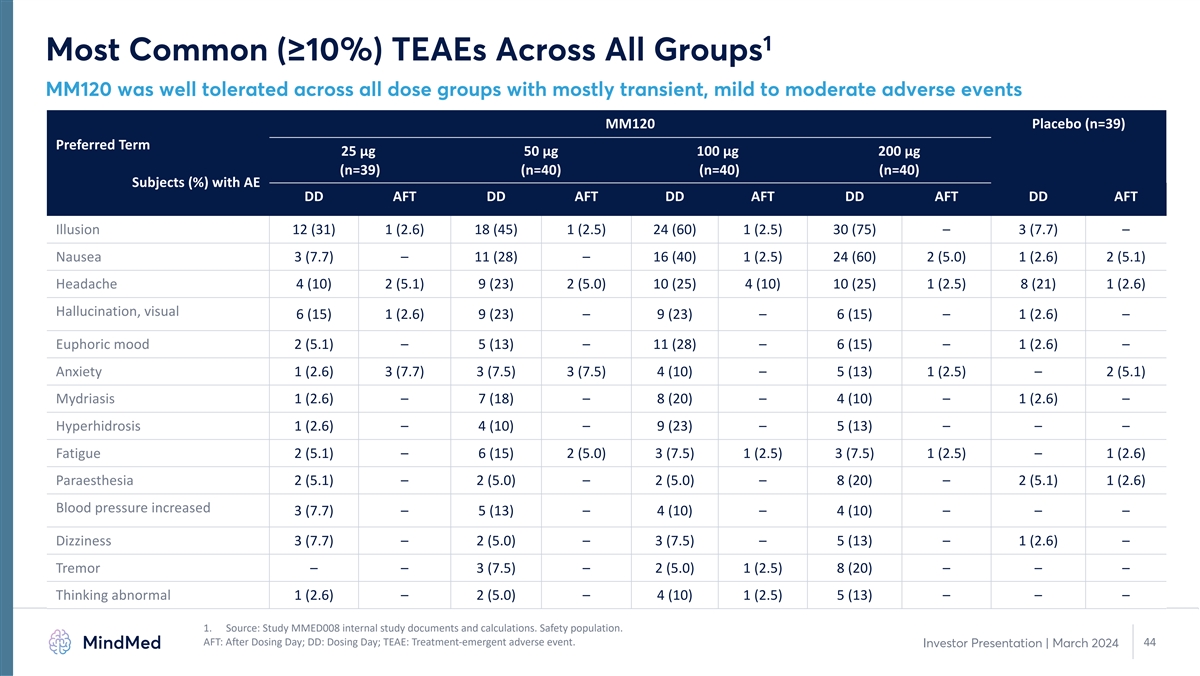
1 Most Common (≥10%) TEAEs Across All Groups MM120 was well tolerated across all dose groups with mostly transient, mild to moderate adverse events MM120 Placebo (n=39) Preferred Term 25 µg 50 µg 100 µg 200 µg (n=39) (n=40) (n=40) (n=40) Subjects (%) with AE DD AFT DD AFT DD AFT DD AFT DD AFT Illusion 12 (31) 1 (2.6) 18 (45) 1 (2.5) 24 (60) 1 (2.5) 30 (75) – 3 (7.7) – Nausea 3 (7.7) – 11 (28) – 16 (40) 1 (2.5) 24 (60) 2 (5.0) 1 (2.6) 2 (5.1) Headache 4 (10) 2 (5.1) 9 (23) 2 (5.0) 10 (25) 4 (10) 10 (25) 1 (2.5) 8 (21) 1 (2.6) Hallucination, visual 6 (15) 1 (2.6) 9 (23) – 9 (23) – 6 (15) – 1 (2.6) – Euphoric mood 2 (5.1) – 5 (13) – 11 (28) – 6 (15) – 1 (2.6) – Anxiety 1 (2.6) 3 (7.7) 3 (7.5) 3 (7.5) 4 (10) – 5 (13) 1 (2.5) – 2 (5.1) Mydriasis 1 (2.6) – 7 (18) – 8 (20) – 4 (10) – 1 (2.6) – Hyperhidrosis 1 (2.6) – 4 (10) – 9 (23) – 5 (13) – – – Fatigue 2 (5.1) – 6 (15) 2 (5.0) 3 (7.5) 1 (2.5) 3 (7.5) 1 (2.5) – 1 (2.6) Paraesthesia 2 (5.1) – 2 (5.0) – 2 (5.0) – 8 (20) – 2 (5.1) 1 (2.6) Blood pressure increased 3 (7.7) – 5 (13) – 4 (10) – 4 (10) – – – Dizziness 3 (7.7) – 2 (5.0) – 3 (7.5) – 5 (13) – 1 (2.6) – Tremor – – 3 (7.5) – 2 (5.0) 1 (2.5) 8 (20) – – – Thinking abnormal 1 (2.6) – 2 (5.0) – 4 (10) 1 (2.5) 5 (13) – – – 1. Source: Study MMED008 internal study documents and calculations. Safety population. AFT: After Dosing Day; DD: Dosing Day; TEAE: Treatment-emergent adverse event. 44 Investor Presentation | March 2024
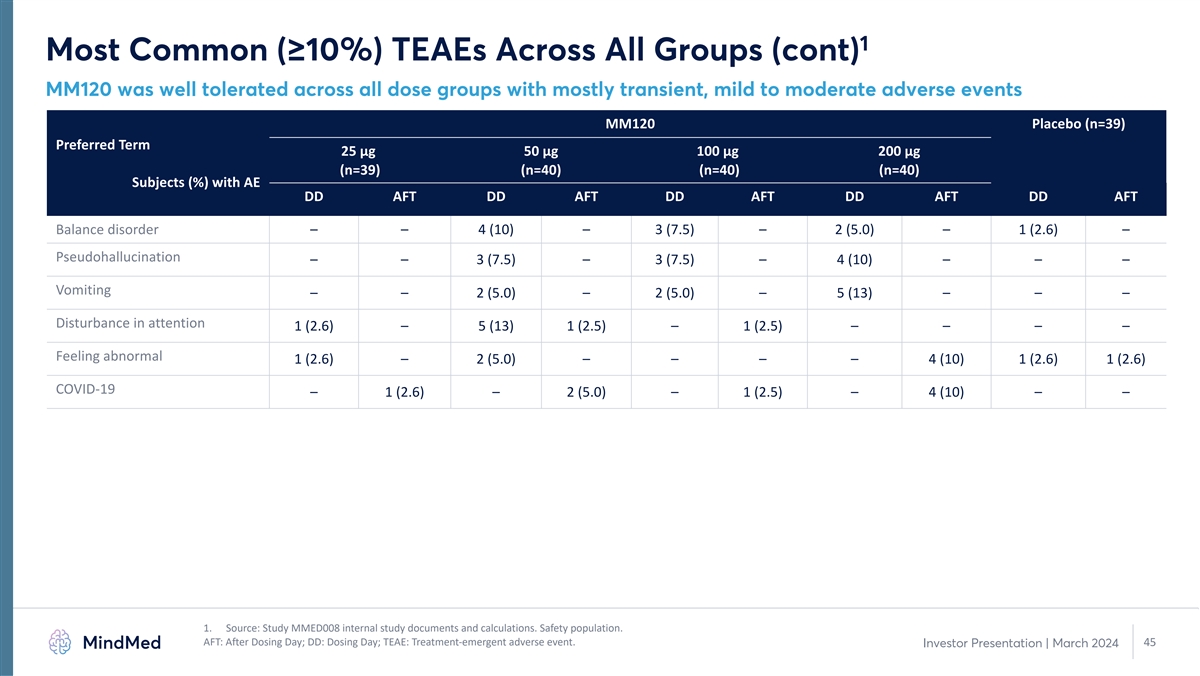
1 Most Common (≥10%) TEAEs Across All Groups (cont) MM120 was well tolerated across all dose groups with mostly transient, mild to moderate adverse events MM120 Placebo (n=39) Preferred Term 25 µg 50 µg 100 µg 200 µg (n=39) (n=40) (n=40) (n=40) Subjects (%) with AE DD AFT DD AFT DD AFT DD AFT DD AFT Balance disorder – – 4 (10) – 3 (7.5) – 2 (5.0) – 1 (2.6) – Pseudohallucination – – 3 (7.5) – 3 (7.5) – 4 (10) – – – Vomiting – – 2 (5.0) – 2 (5.0) – 5 (13) – – – Disturbance in attention 1 (2.6) – 5 (13) 1 (2.5) – 1 (2.5) – – – – Feeling abnormal 1 (2.6) – 2 (5.0) – – – – 4 (10) 1 (2.6) 1 (2.6) COVID-19 – 1 (2.6) – 2 (5.0) – 1 (2.5) – 4 (10) – – 1. Source: Study MMED008 internal study documents and calculations. Safety population. AFT: After Dosing Day; DD: Dosing Day; TEAE: Treatment-emergent adverse event. 45 Investor Presentation | March 2024