Exhibit 99.1

MindMed Corporate Over April 2022
Exhibit 99.1

MindMed Corporate Over April 2022

Disclaimer This presentation (the “Presentation”) has been prepared by Mind Medicine (MindMed) Inc. (“MindMed” or the “Company”) solely for informational purposes. None of MindMed, its affiliates or any of their respective employees, directors, officers, contractors, advisors, members, successors, representatives or agents makes any representation or warranty as to the accuracy or completeness of any information contained in this Presentation and shall have no liability for any representations (expressed or implied) contained in, or for any omissions from, this Presentation. This presentation shall not constitute an offer, nor a solicitation of an offer, of the sale or purchase of securities. This Presentation does not constitute an offering of securities of MindMed and under no circumstances is it to be construed as a prospectus or advertisement or public offering of securities. Any trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of the products or services of MindMed. Any amounts are in USD unless otherwise noted. MindMed’s securities have not been approved or disapproved by the SEC or by any state, provincial or other securities regulatory authority, nor has the SEC or any state, provincial or other securities regulatory authority passed on the accuracy or adequacy of this Presentation. Any representation to the contrary is a criminal offense. Cautionary Note Regarding Forward-Looking Statements This Presentation contains, and our officers and representatives may from time to time make, “forward-looking statements” within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995 and other applicable securities laws. Forward- looking statements can often, but not always, be identified by words such as “plans”, “expects”, “is expected”, “budget”, “scheduled”, “estimates”, “forecasts”, “intends”, “anticipates”, will”, “projects”, or “believes” or variations (including negative variations) of such words and phrases, or statements that certain actions, events, results or conditions “may”, “could”, “would”, “might” or “will” be taken, occur or be achieved, and similar references to future periods. Except for statements of historical fact, examples of forward-looking statements include, among others, statements pertaining to the development and commercialization of any medicine or treatment, or the efficacy of either of the foregoing, the success and timing of our development activities, the success and timing of our planned clinical trials, our ability to meet the milestones set forth herein; the likelihood of success of any clinical trials or of obtaining FDA or other regulatory approvals, the likelihood of obtaining patents or the efficacy of such patents once granted, and the potential for the markets that MindMed is anticipating to access. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the economy and other future conditions as of the date of this Presentation. While we consider these assumptions to be reasonable, the assumptions are inherently subject to significant business, social, economic, political, regulatory, competitive and other risks and uncertainties that are difficult to predict and many of which are outside of our control, and our actual results and financial condition may differ materially from those indicated in the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause our actual results and financial condition to differ materially from those indicated in the forward-looking statements include, among others, the following: our ability to raise capital to complete its plans and fund its studies; the medical and commercial viability of the contemplated medicines and treatments being developed; our ability to raise additional capital in the future as we continue to develop our products; our history of negative cash flows; our limited operating history; incurrence of future losses; availability of additional capital; lack of revenue; compliance with laws and regulations; difficulty associated with research and development; risks associated with clinical trials or studies; heightened regulatory scrutiny; early stage product development; clinical trial risks; regulatory approval processes; novelty of the psychedelic inspired medicines industry; as well as those risk factors discussed or referred to throughout the “Risk Factors” sections of our most recently filed Annual Report on Form 10-K filed with the Securities and Exchange Commission (the “SEC”) and in other filings we make in the future with the SEC and the securities regulatory authorities in all provinces and territories of Canada, available under the Company’s profile on SEDAR at www.sedar.com. Any forward-looking statement made by us in this Presentation is based only on information currently available to us and speaks only as of the date on which it is made. MindMed undertakes no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise. Cautionary Note Regarding Regulatory Matters The United States federal government regulates drugs through the Controlled Substances Act. The Company works with a non-hallucinogenic synthetic derivative of the psychedelic substance ibogaine, known as “18-MC”, which is a synthetic organic molecule designed around a common coronaridine chemical backbone. 18-MC is not a Schedule I substance in the United States and the Company does not foresee it becoming a Schedule I substance due to its non-hallucinogenic properties. While the Company is focused on programs using psychedelic inspired compounds and classic psychedelics, the Company does not have any direct or indirect involvement with the illegal selling, production or distribution of any substances in the jurisdictions in which it operates. The Company is a neuro-pharmaceutical drug development company and does not deal with psychedelic substances except within laboratory and clinical trial settings conducted within approved regulatory frameworks. The Company’s products will not be commercialized prior to applicable regulatory approval, which will only be granted if clinical evidence of safety and efficacy for the intended uses is successfully developed.] Market and Industry Data This Presentation by includes market and industry data that has been obtained from third party sources, including industry publications. MindMed believes that the industry data is accurate and that the estimates and assumptions are reasonable, but there is no assurance as to the accuracy or completeness of this data. Third party sources generally state that the information contained therein has been obtained from sources believed to be reliable, but there is no assurance as to the accuracy or completeness of included information. Although the data is believed to be reliable, MindMed has not independently verified any of the data from third party sources referred to in this Presentation or ascertained the underlying economic assumptions relied upon by such sources. References in this Presentation to research reports or to articles and publications should be not construed as depicting the complete findings of the entire referenced report or article. MindMed does not make any representation as to the accuracy of such information. MindMed Corporate Overview | April 2022 2

Business Highlights Our mission is to deliuer on the therapeutic potential of psychedelics and other nouel targets to treat brain health disorders • Leader in developing psychedelic product candidates to treat brain health disorders • Diversified pipeline of clinical programs targeting significant unmet medical needs • IP and R&D strategies to maximize market exclusivity and protection • Leveraging decades of research on clinical and preclinical potential of product candidates • Industry-leading expertise in drug and digital medicine development and commercialization • Fully funded through key clinical readouts and into 2024 MindMed Corporate Overview | April 2022 3
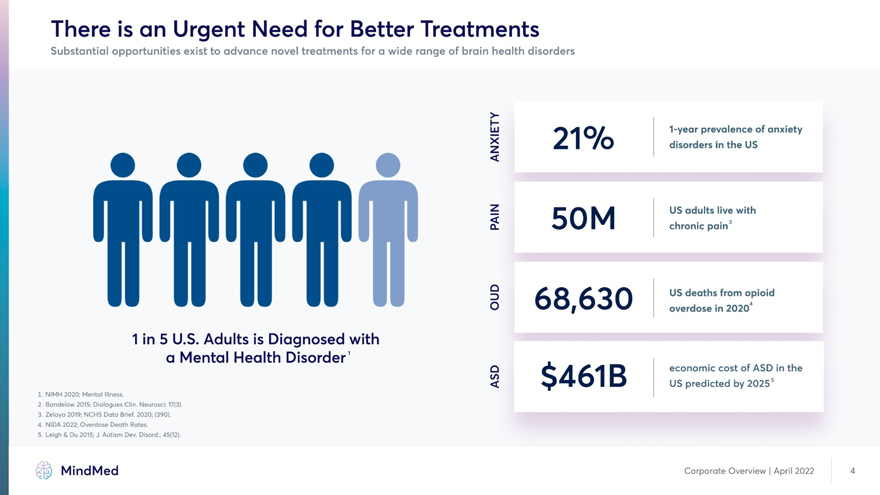
There is an Urgent Need for Better Treatments Substantial opportunities exist to advance novel treatments for a wide range of brain health disorders >- â–¡J 1-year prevalence of anxiety 7 | /O disorders in the US2 • • • • • ’ W W W W 15 0 M “”” Q people in the US overdosed W W W W Ww Q on opioids in 2020 1 in 5 U.S. Adults is Diagnosed with a Mental Health Disorder . Q A economic cost of ASD in the < I Ev US predicted by 2O255 1. Mental Illness 2020; NIMH 2. Bandelow 2015; Dialogues Clin. Neurosci; 17(3) 3. Zelaya 2019; NCHS Data Brief. 2020;(390) 4. Overdose Death Rates 2022, February 1; NIDA 5. Leigh & Du 2015; J. Autism Dev. Disord. 45(12) MindMed Corporate Overview | April 2022 4
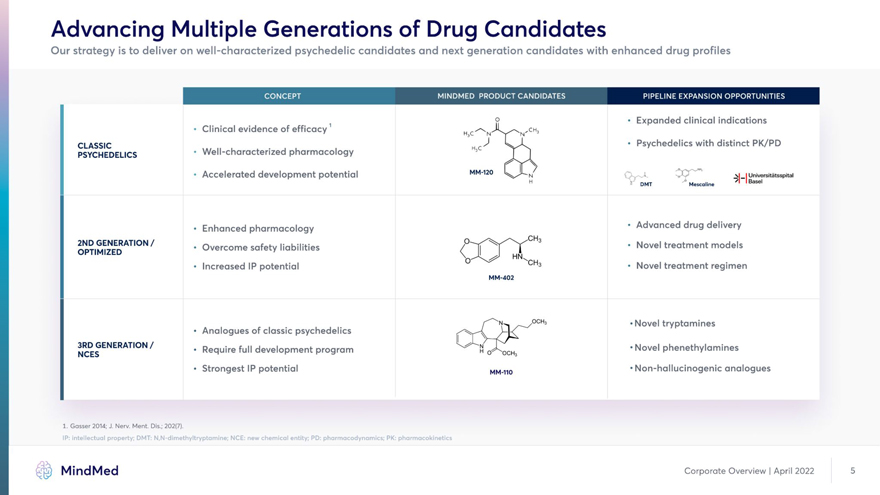
Advancing Multiple Generations of Drug Candidates Our strategy is to deliver on well-characterized psychedelic candidates and next generation candidates with enhanced drug profiles 1 o • Expanded clinical indications • Clinical evidence of efficacy /H3 * N N CLASSIC HC^ ‘ Psychedelics with distinct PK/PD PSYCHEDELICS ’ Well-characterized pharmacology • Accelerated development potential MM-120 i I Universitatsspital DMT Mescaline ‘83561 • Enhanced pharmacology ‘ Advanced drug delivery OPTIM^ZEEDATI°N/ ‘ Overcome safety liabilities ‘ Novel treatment models • Increased IP potential 3 • Novel treatment regimen MM-402 0CH3 • Novel tryptamines • Analogues of classic psychedelics NCES ENERAT,°N/ ’ Recluire ful1 development program oAicnj ’Novel Phenethylamines • Strongest IP potential MM-HO • Non-hallucinogenic analogues 1. Gasser 2014; J. Nerv. Merit. Dis.; 202(7). IP: intellectual property; DMT: N,N-dimethyltryptamine; NCE: new chemical entity; PD: pharmacodynamics; PK: pharmacokinetics MindMed Corporate Overview | April 2022 5
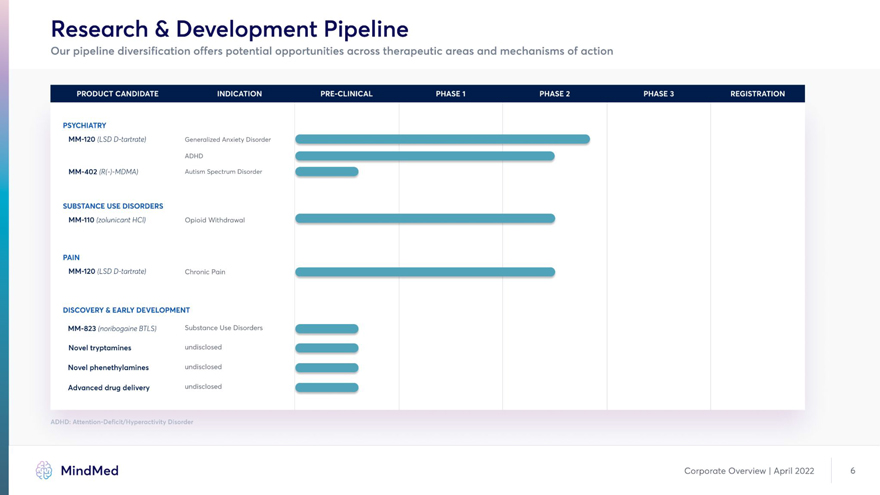
Research & Development Pipeline Our pipeline diversification offers potential opportunities across therapeutic areas and mechanisms of action PSYCHIATRY ADHD MM-402 (R(-)-MDMA) Autism Spectrum Disorder SUBSTANCE USE DISORDERS MM-110 (zolunicant HCI) Opioid Withdrawal PAIN DISCOVERY & EARLY DEVELOPMENT MM-823 (noribogaine BTLS) Substance Use Disorders Novel tryptamines undisclosed Novel phenethylamines undisclosed Advanced drug delivery undisclosed ADHD: Attention-Deficit/Hyperactivity Disorder MindMed Corporate Overview | April 2022 6
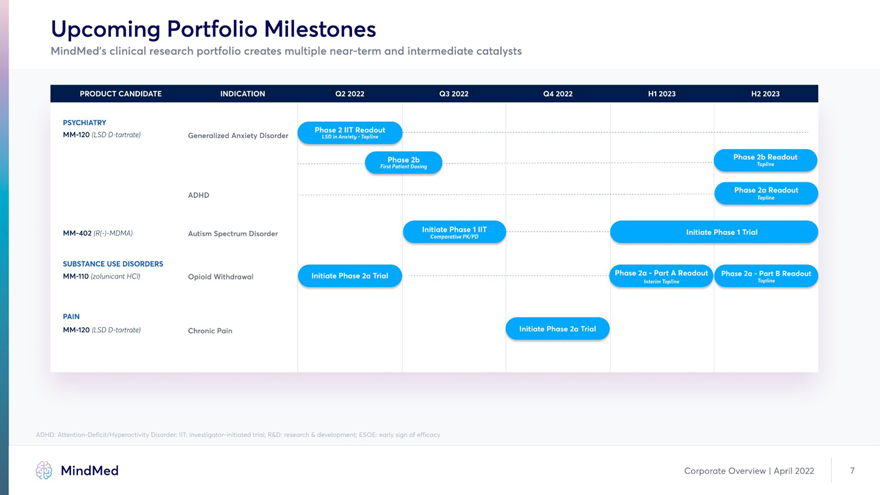
Upcoming Portfolio Milestones MindMed’s clinical research portfolio creates multiple near-term and intermediate catalysts PSYCHIATRY MM-120 (LSD D-tartrate) Generalized Anxiety Disorder S ADHD MM-402 (R(-)-MDMA) Autism Spectrum Disorder SUBSTANCE USE DISORDERS /â– HWWWWIk MM-110 (zolunicant HCI) Opioid Withdrawal PAIN MM-120 (LSD D-tartrate) Chronic Pain ADHD: Attention-Deficit/Hyperactivity Disorder; IIT: investigator-initiated trial; R&D: research & development; ESOE: early sign of efficacy MindMed Corporate Overview | April 2022 7
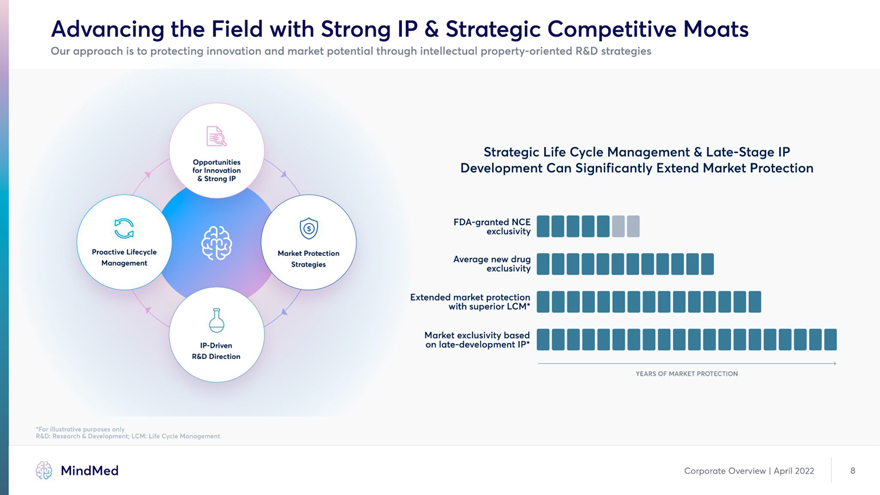
Advancing the Field with Strong IP & Strategic Competitive Moats Our approach is to protecting innovation and market potential through intellectual property-oriented R&D strategies Strategic Life Cycle Management & Late-Stage IP for Innovation Development Can Significantly Extend Market Protection & Strong IP / FDA-granted NCE I I exclusivity Proactive Lifecycle Market Protection Extended market protection | _ \ k with superior LCM« Market exclusivity based I IP-Driven on late-development IP* | R&D Direction X. s’ YEARS OF MARKET PROTECTION *For illustrative purposes only R&D: Research & Development; LCM: Life Cycle Management MindMed Corporate Overview | April 2022 8

LSD-Anxiety Topline Readout Q2 2022 | Phase 2 (IIT) GAD First Patient Dosing >1 I 2022 phase Chronic Pain Study Initiation Q4 2022 | Phase 2 ESOE MindMed Corporate Overview | April 2022 9
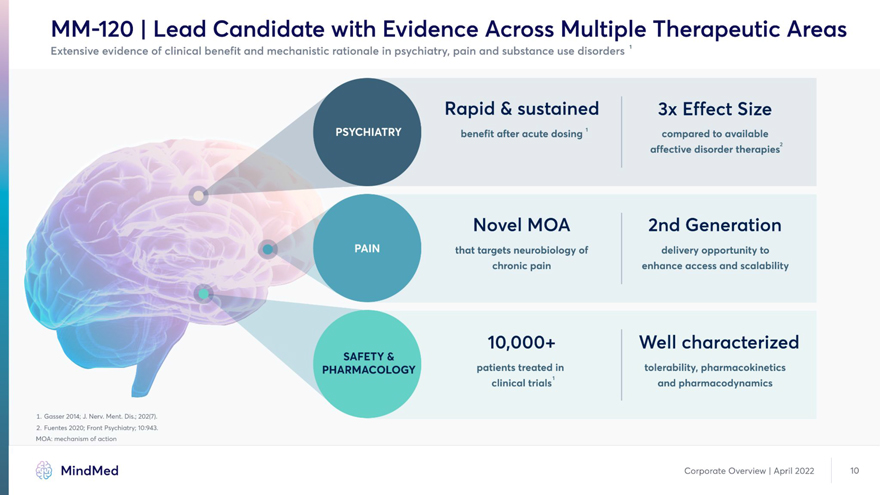
MM-120 | Lead Candidate with Evidence Across Multiple Therapeutic Areas Extensive evidence of clinical benefit and mechanistic rationale in psychiatry, pain and substance use disorders 1 Rapid & sustained 3x Effect Size benefit after acute dosing1 compared to available affective disorder therapies2 Novel MOA 2nd Generation that targets neurobiology of delivery opportunity to enhance access 10,000+ Well characterized SAFETY & PHARMACOLOGY patients treated in tolerability, pharmacokinetics clinical trials1 and pharmacodynamics 1. Gasser 2014; J. Nerv. Ment. Dis.; 202(7). 2. Fuentes 2020; Front Psychiatry; 10:943. MOA: mechanism of action MindMed Corporate Overview | April 2022 10
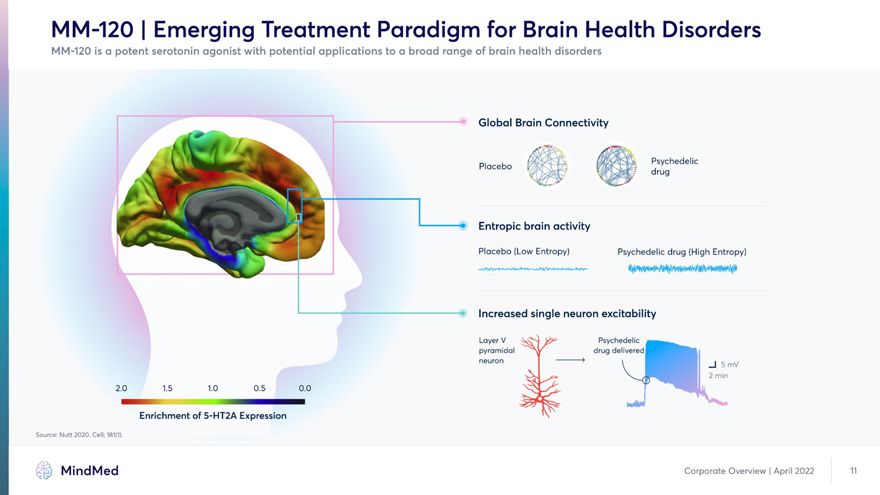
MM-120 | Emerging Treatment Paradigm for Brain Health Disorders Novel mechanisms of action with transdiagnostic applicability Global Brain Connectivity / Placebo (Low Entropy) Psychedelic drug (High Entropy) I Increased single neuron excitability Layer V v-fj & Psychedelic pyramidal A drug delivered neuron > t _| 5 mV 2.0 1.5 1.0 0.5 0.0 Source: Nutt 2020. Cell; 181(1). Enrichment of 5-HT2A Expression MindMed Corporate Overview | April 2022 11

MM-120 | Legacy of LSD Clinical Research in Psychiatric Disorders Building on decades of clinical research on LSD in anxiety and depression 21 STUDIES Anxiety, depression & 512 patients Up to 95% reduction in PRIOR T019741 ‘neuroses’ symptoms GASSER 20142 Anxiety in 12 patients Effect size of 1.1 with durable terminal illness reductions in anxiety at 1 year ’ LSD-ASSIST STUDY Anxiety 41 patients Topline results expected in Q2 2022 Rucker Gasser MindMed Corporate Overview | April 2022 12
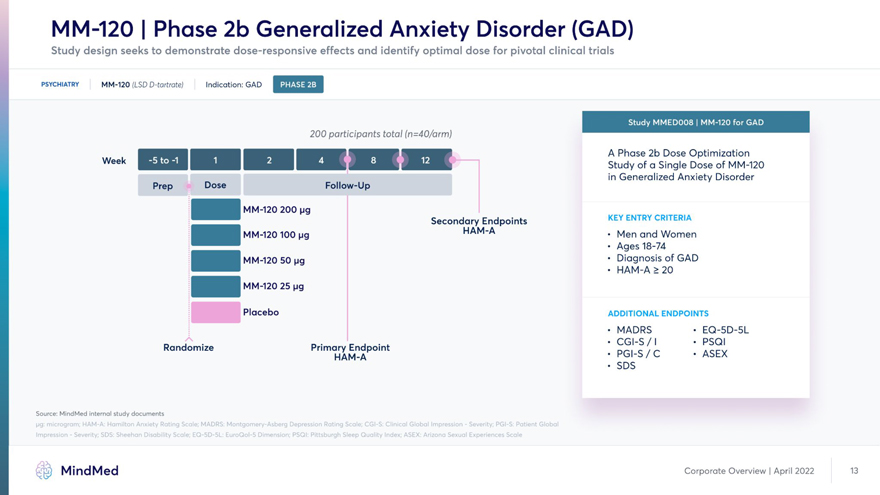
MM-120 | Phase 2b Generalized Anxiety Disorder (GAD) Study design seeks to demonstrate dose-responsive effects and identify optimal dose for pivotal clinical trials PSYCHIATRY MM-120 (LSD D-tartrate) Indication: GAD 200 participants total (n=40/arm) HMHM M H ^B Phase Dose Week | J |^^B^^B ^^1 ‘ ^B^B Study a Dose of MM-120 in Generalized Anxiety Disorder Prep Dose Follow-Up I MM-120 200 pg Secondary Endpoint KEY ENTRY CRITER,A I MM-120 100 pg HAM-A . j^en anc| Women • Ages 18-74 I MM-120 50 pg * Diagnosis of GAD • HAM-A >20 | MM-120 25 pg Placebo ADDITIONAL ENDPOINTS • MADRS • EQ-5D-5L • CGI-S/I • PSQI Randomize Primary Endpoint • PGI-S / C • ASEX HAM-A . SDS Source: MindMed internal study documents pg: microgram; HAM-A: Hamilton Anxiety Rating Scale; MADRS: Montgomery-Asberg Depression Rating Scale; CGI-S: Clinical Global Impression—Severity; PGI-S: Patient Global Impression—Severity; SDS: Sheehan Disability Scale; EQ-5D-5L: EuroQol-5 Dimension; PSQI: Pittsburgh Sleep Quality Index; ASEX: Arizona Sexual Experiences Scale MindMed Corporate Overview | April 2022 13
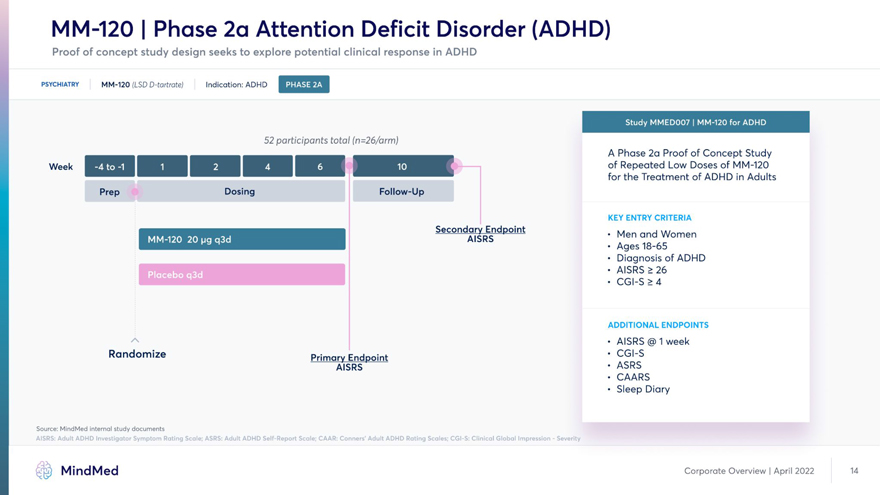
MM-120 | Phase 2a Attention Deficit Disorder (ADHD) Proof of concept study design seeks to explore potential clinical response in ADHD PSYCHIATRY MM-120 (LSD D-tartrate) Indication: ADHD 52 participants total (n=26/arm) A Phase 2a Proof of Concept Study Week LOW ^OSES for the Treatment of ADHD in Adults Prep Dosing Follow-Up KEY ENTRY CRITERIA Secondary ^Endpoint . Men and Women • Ages • Diagnosis of ADHD • AISRS > 26 • CGI-S > 4 ADDITIONAL ENDPOINTS • AISRS @ 1 week Randomize Primary Endpoint ’ CGI-S AISRS • ASRS • CAARS • Sleep Diary Source: MindMed internal study documents AISRS: Adult ADHD Investigator Symptom Rating Scale; ASRS: Adult ADHD Self-Report Scale; CAAR: Conners’ Adult ADHD Rating Scales; CGI-S: Clinical Global Impression—Severity MindMed Corporate Overview | April 2022 14
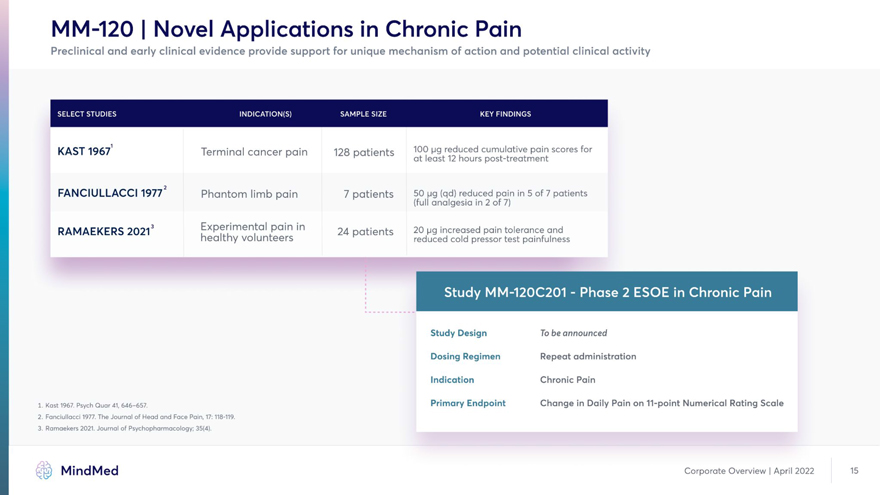
MM-120 | Novel Applications in Chronic Pain Preclinical and early clinical evidence provide support for unique mechanism of action and potential clinical activity KAST 1967 Terminal cancer pain 128 patients 100 ug reduced cumulative pain scores for at least 12 hours post-treatment 2 FANCIULLACCI 1977 Phantom limb pain 7 patients 50 pg (qd) reduced pain in 5 of 7 patients (full analgesia in 2 of 7) RAMAEKERS 2O213 Experimental pain in 24 patients 20 pg increased pain tolerance and healthy volunteers reduced cold pressor test painfulness i Study Design To be announced Dosing Regimen Repeat administration Indication Chronic Pain 1. Kast 1967. Psych Quar 41,646-657. Primary Endpoint Change in Daily Pain on 11-point Numerical Rating Scale 2. Fanciullacci 1977. The Journal of Head and Face Pain, 17:118-119. 3. Ramaekers 2021. Journal of Psychopharmacology; 35(4). MindMed Corporate Overview | April 2022 15

Phase 1 Topline Data Readout Q2 2022 | Phase 1 Opioid W/D Study Initiation Q2 2022 | Phase 2a Opioid W/D ESOE Readout Q4 2022 | Phase 2a (Part A) MindMed Corporate Overview | April 2022 16
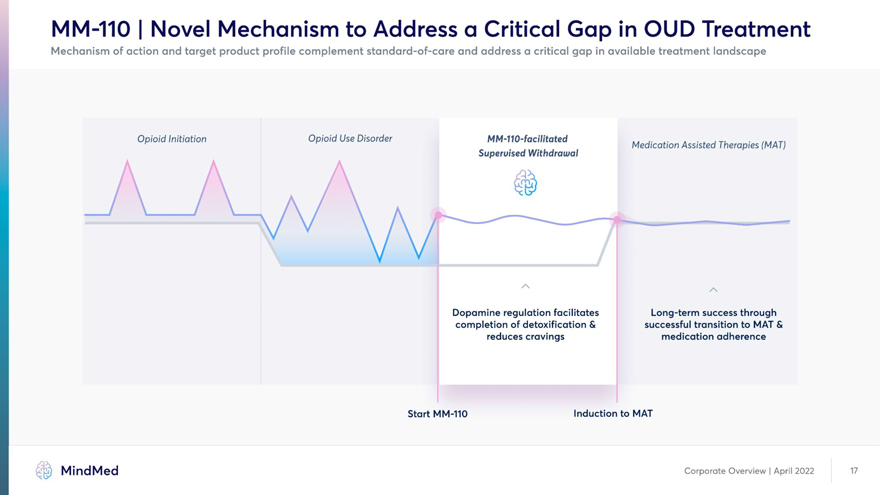
MM-110 | Novel Mechanism to Address a Critical Gap in OUD Treatment Mechanism of action and target product profile complement standard-of-care and address a critical gap in available treatment landscape Opioid Initiation Opioid Use Disorder MM-110-facilitated Medication Assisted Therapies (MAT) Superuised Withdrawal Dopamine regulation facilitates Long-term success through completion of detoxification & successful transition to MAT & reduces cravings medication adherence Start MM-110 Induction to MAT MindMed Corporate Overview | April 2022 17
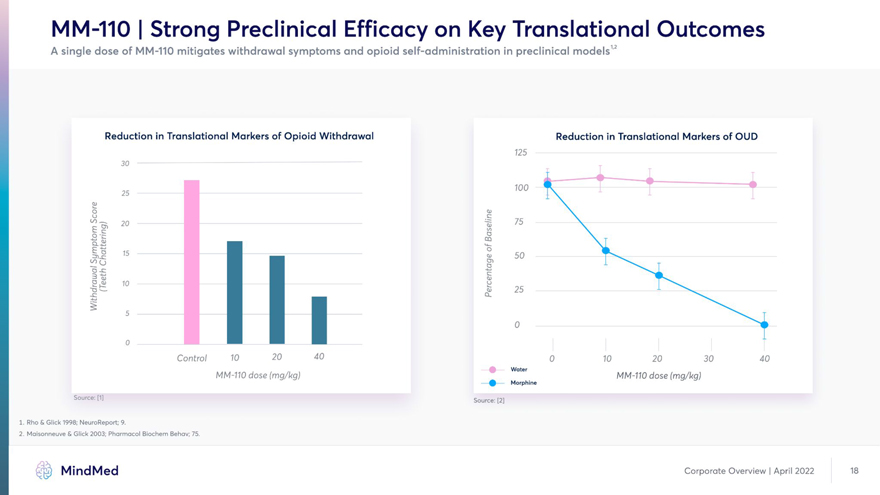
MM-110 | Strong Preclinical Efficacy on Key Translational Outcomes A single dose of MM-110 mitigates withdrawal symptoms and opioid self-administration in preclinical models12 Reduction in Translational Markers of Opioid Withdrawal Reduction in Translational Markers of OUD 125 30 20 I Control 10 20 40 Q 10 20 30 40 Water MM-110 dose (mg/kg) MM-110 dose (mg/kg) 4) Morphine Source: W Source: [2] 1. Rho & Glick 1998; NeuroReport; 9. 2. Maisonneuve & Glick 2003; Pharmacol Biochem Behav; 75.
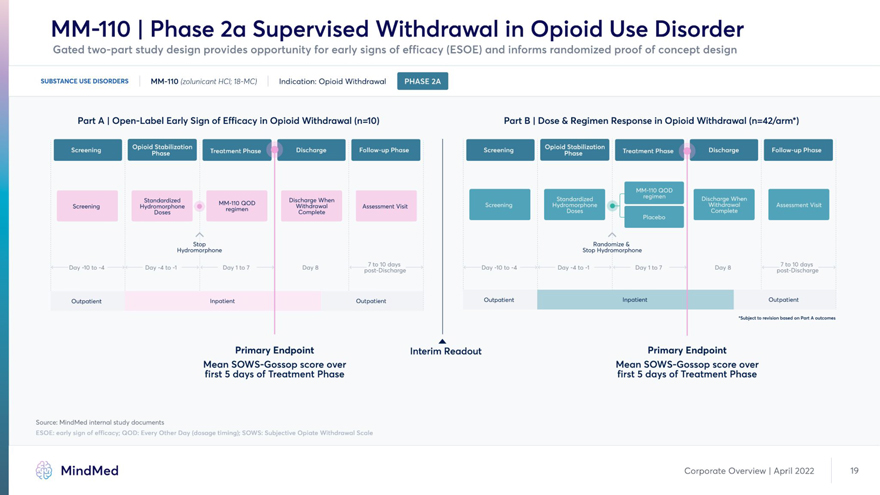
MindMed Corporate Overview | April 2022 1J MM-110 | Phase 2a Supervised Withdrawal in Opioid Use Disorder Gated two-part study design provides opportunity for early signs of efficacy (ESOE) and informs randomized proof of concept design SUBSTANCE USE DISORDERS MM-110 (zolunicant HCI; 18-MC) Indication: Opioid Withdrawal Part A | Open-Label Early Sign of Efficacy in Opioid Withdrawal (n=10) Part B | Dose & Regimen Response in Opioid Withdrawal (n=TBD*) Standardized MM-110 QOD Discharge When H H Screening Hydromorphone Withdrawal Assessment Visit Doses re9’men Complete Stop Randomize & Hydromorphone Stop Hydromorphone Day-10 to-4 Day-4 to-1 Day 1 to 7 Day 8 poshDischoVge Day-10 to-4 Day-4 to-1 Day 1 to 7 Day 8 port-lSchJrge Outpatient Inpatient Outpatient Outpatient Inpatient Outpatient ‘Subject to revision based on Port A outcomes Primary Endpoint Interim Readout Primary Endpoint Mean SOWS-Gossop score over Mean SOWS-Gossop score over first 5 days of Treatment Phase first 5 days of Treatment Phase Source: MindMed internal study documents ESOE: early sign of efficacy; QOD: Every Other Day (dosage timing); SOWS: Subjective Opiate Withdrawal Scale MindMed Corporate Overview | April 2022 19

Key Milestones MM_402 EEEES
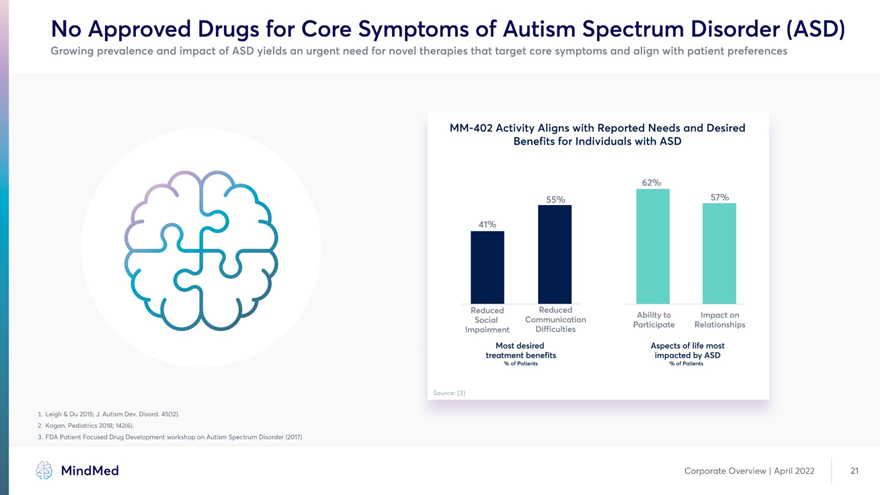
No Approved Drugs for Core Symptoms of Autism Spectrum Disorder (ASD) Growing prevalence and impact of ASD yields an urgent need for novel therapies that target core symptoms and align with patient preferences MM-402 Activity Aligns with Reported Needs and Desired Benefits for Individuals with ASD I I 1J Reduced Reduced A -I.- Ability to Impact on /v Social Communication Participate Relationships Impairment Difficulties Most desired Aspects of life most treatment benefits impacted by ASD % of Patients % of Patients Source: [3]
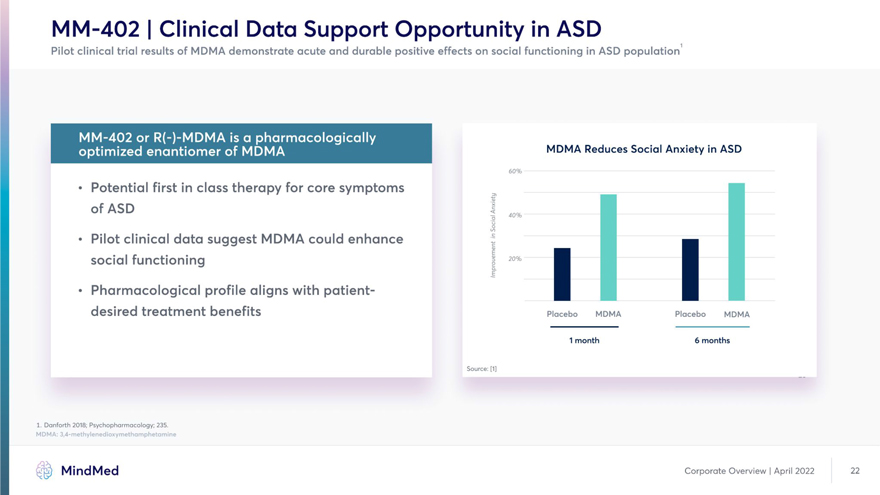
Leigh & Du 2015; J. Autism Dev. Disord. 45(12). 2. Kogan, Pediatrics 2018; 142(6). 3. FDA Patient Focused Drug Development workshop on Autism Spectrum Disorder (2017) MindMed Corporate Overview | April 2022 21 MM-402 | Clinical Data Support Opportunity in ASD Pilot clinical trial results of MDMA demonstrate acute and durable positive effects on social functioning in ASD population1 MDMA Reduces Social Anxiety in ASD 60% • Potential first in class therapy for core symptoms of ASD H ~ 40% • Pilot clinical data suggest MDMA could enhance | social functioning H H H H • Pharmacological profile aligns with patient- H desired treatment benefits placebo MDMA placebo MDMA 1 month 6 months Source: [1] 1. Danforth 2018; Psychopharmacology; 235. MDMA: 3,4-methylenedioxymethamphetamine MindMed Corporate Overview | April 2022 22
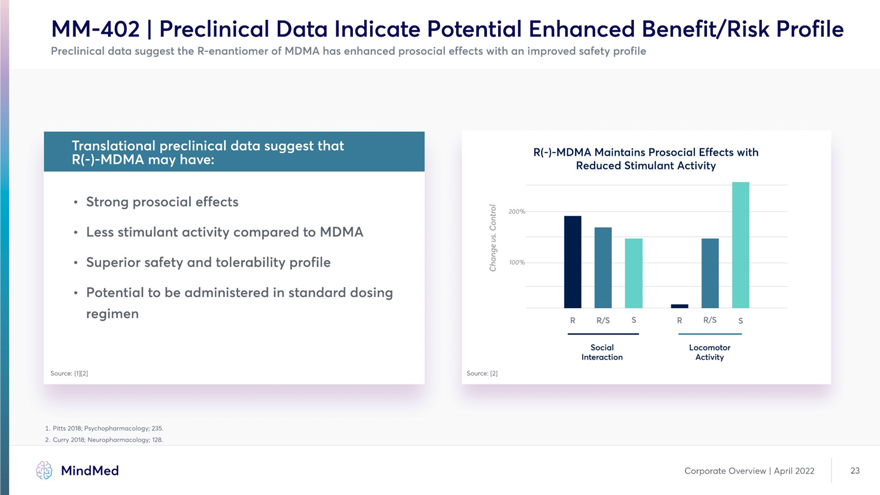
MM-402 | Preclinical Data Indicate Potential Enhanced Benefit/Risk Profile Preclinical data suggest the R-enantiomer of MDMA has enhanced prosocial effects with an improved safety profile K| f kit- Ip fcl I j I R(-)-MDMA Maintains Prosocial Effects with • Strong prosocial effects 2 200% • Less stimulant activity compared to MDMA H M • Superior safety and tolerability profile i M • Potential to be administered in standard dosing H re9imen R R/S s T R/S s Social Locomotor Interaction Activity Source: {1][2J Source: [2] 1. Pitts 2018; Psychopharmacology; 235. 2. Curry 2018; Neuropharmacology; 128. MindMed Corporate Overview | April 2022 23

Collaborations & Early R&D
External Collaborations Accelerate Discovery & Development Leveraging key partnerships and collaborations to accelerate drug discovery and de-risk clinical development DISCOVERY & BRAIN TARGETED LIPOSOMES RAPID DATA GENERATION & LEAD OPTIMIZATION (BTLS) CLINICAL CONCEPT TESTING MindShift wnextage ^reit5tsspital Compounds MindMed Corporate Overview | April 2022 25
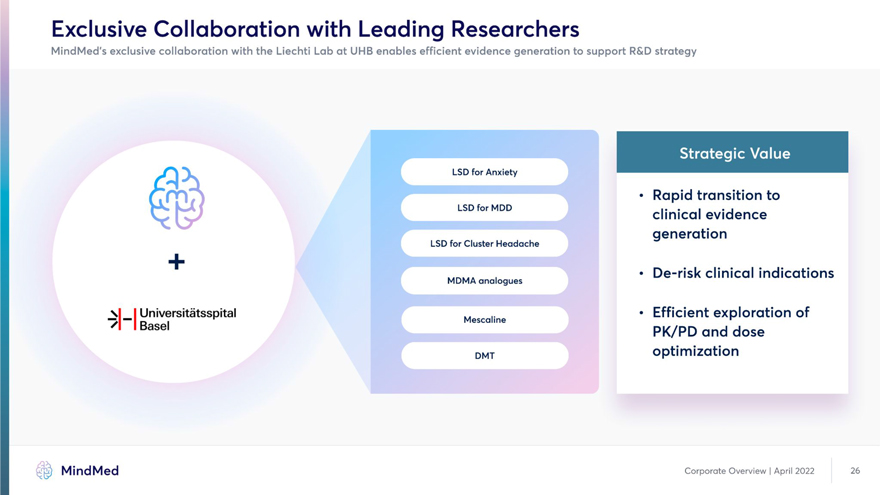
Exclusive Collaboration with Leading Researchers MindMed’s exclusive collaboration with the Liechti Lab at UHB enables efficient evidence generation to support R&D strategy for (7m7) • Rapid transition to LSDforMDD clinical evidence generation LSD for Cluster Headache De-risk clinical indications MDMA analogues \| | Universitatsspital • Efficient exploration of 1 1 Basel PK/PD and dose DMT optimization MindMed Corporate Overview I April 2022 26

Digital Medicine

Digital Unlocks Potential Opportunities Throughout the Product Lifecycle Generating data, insights, models, and tools from early development through market management Preclinical Research IND & Phases 1—3 Drug Launch Enhancement and Lifecycle Management • Deep Digital Diagnoses • Decision Support • Surveillance & Registries • Drug-Device Combinations • Decentralized Trials • Predictive Intervention • Remote Management • Lifecycle Enhancement • Advanced Analytics • Patient Engagement • HEOR • Efficient Phase 4 Research HEOR: health economics and outcomes research MindMed Corporate Overview | April 2022 28
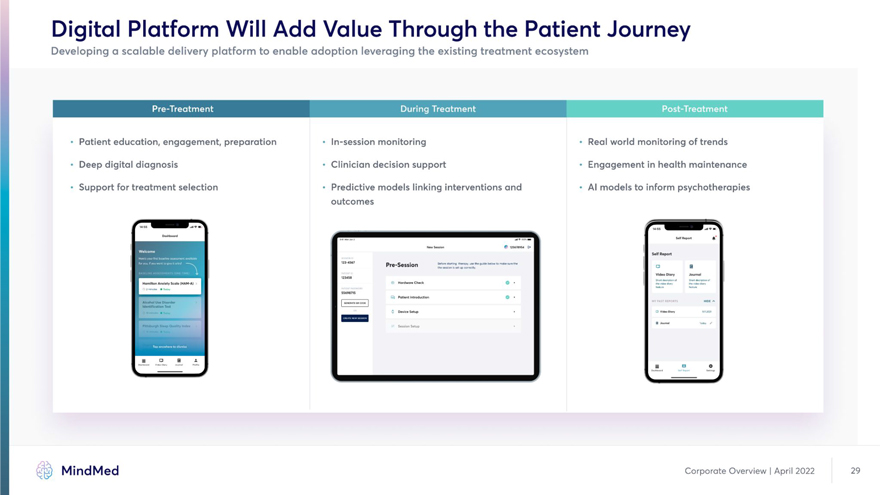
Digital Platform Will Add Value Through the Patient Journey Developing a scalable delivery platform to enable adoption leveraging the existing treatment ecosystem • Patient education, engagement, preparation • In-session monitoring • Real world monitoring of trends • Deep digital diagnosis • Clinician decision support • Engagement in health maintenance • Support for treatment selection • Predictive models linking interventions and • Al models to inform psychotherapies outcomes __ Dashboard . I I PeporS A l| ] Hew Session Soil Report “ Pre-Session | o a 1 Video Diary Journal 12345® Hamilton Anxiety Scale (HAM-A) Hardware Check Q | “M /SKOU-. |H I’.iWnOS O Ttttcrf ‘ Patient introduction Q AJcabo) UM Disorder I OCNMVKMCOX ] I MV FA5T BEPO“TS H,oe * Identification Test H Pittsburgh Heep Quollty Indei I Session 5etup I MindMed Corporate Overview | April 2022 29
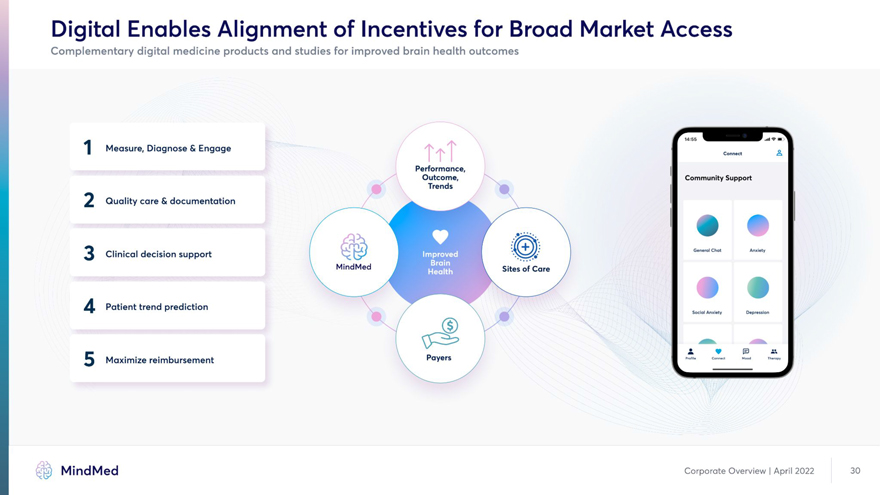
Digital Enables Alignment of Incentives for Broad Market Access Complementary digital medicine products and studies for improved brain health outcomes J Measure, Diagnose & Engage Connect A Performance, 2 Outcome, Community Support Trends i \ / Quality care & documentation I 1 • • , cl.. ld.. rt aUl & i Clinical decision support < Cx d - . 9 # / \ Social Anxiety Depression — X v o -• Q Maximize reimbursement Payers MindMed Corporate Overview | April 2022 30
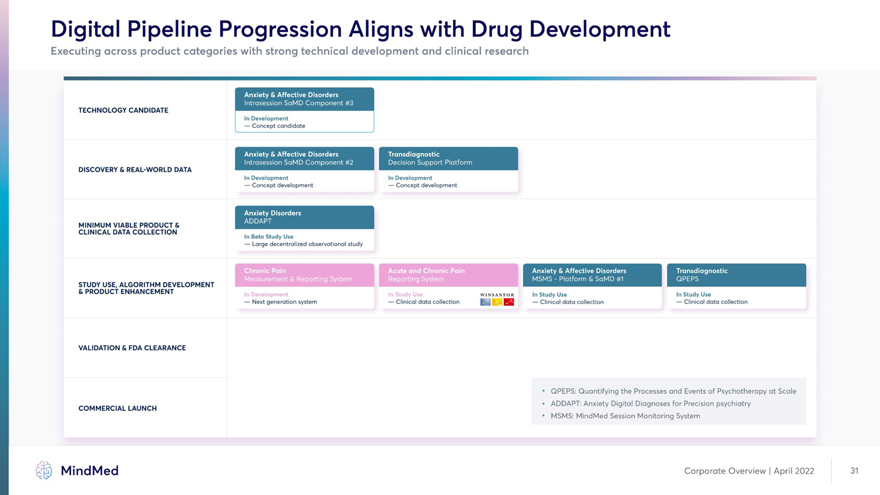
Digital Pipeline Progression Aligns with Drug Development Executing across product categories with strong technical development and clinical research TECHNOLOGY CANDIDATE In Development — Concept candidate DISCOVERY & REAL-WORLD DATA In Development In Development — Concept development — Concept development MINIMUM VIABLE PRODUCTS KaMM CLINICAL DATA COLLECTION In Beta Study Use — Large decentralized observational study STUDY USE, ALGORITHM DEVELOPMENT BlIliM & PRODUCT ENHANCEMENT WINSANTOR In Study Use In Study Use — Next generation system — Clinical data collection i K — Clinical data collection — Clinical data collection VALIDATION & FDA CLEARANCE • QPEPS: Quantifying the Processes and Events of Psychotherapy at Scale COMMERCIAL LAUNCH * ADDAPT: Anxiety Digital Diagnoses for Precision psychiatry • MSMS: MindMed Session Monitoring System MindMed Corporate Overview | April 2022 31

Corporate Information
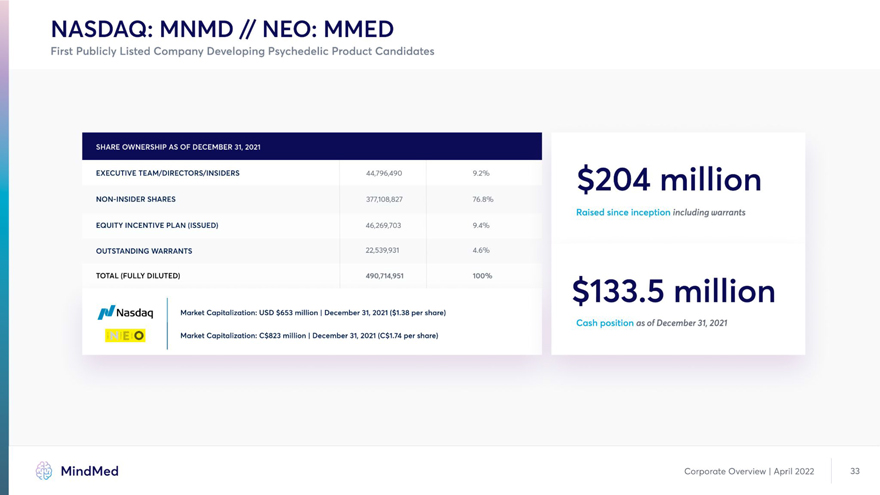
NASDAQ: MNMD // NEO: MMED First Publicly Listed Company Developing Psychedelic Product Candidates EXECUTIVE TEAM/DIRECTORS/INSIDERS 44,796,490 9.2% O A ~ ! I I J million NON-INSIDER SHARES 377,108,827 76.8% Raised since inception including warrants EQUITY INCENTIVE PLAN (ISSUED) 46,269,703 9.4% OUTSTANDING WARRANTS 22,539,931 4.6% TOTAL (FULLY DILUTED) 490,714,951 100% . $133.5 million N3Sd3C] Market Capitalization: USD $653 million | December 31, 2021 ($1.38 per share) Cash position as of December 31, 2021 O Market Capitalization: C$823 million I December 31, 2021 (C$1.74 per share)

MindMed Corporate Overview | April 2022 33 Leadership: Leading Expertise in Innovative Drug & Digital Development Robert Barrow If Miri Halperin Wernli, PhD Cynthia Hu, JD Rob is an accomplished pharmaceutical executive and clinical pharmacologist with Miri co-founded Creso Pharma, a cannabis company, Cynthia joined in December 2021 as Chief Legal Officer & Corporate over a decade of experience leading drug development programs in a variety of and listed the company on the Australian Stock exchange (ASX) in Secretary. Previously, from 2009-2021, she served as COO, General Counsel disease areas. Mr. Barrow previously served as Director of Drug Development & October 2016. Prior to founding Creso Pharma Dr. Halperin Wernli worked in & Secretary at CASI Pharmaceuticals, Inc. and, from 2006 to 2009, as VP, General Discovery at Usona Institute, where he oversaw preclinical, clinical and regulatory clinical psychiatry in Swiss academic hospital settings and then held various global Counsel, of its predecessor, EntreMed, Inc. Prior to that, she served as senior associate development efforts for all of Usona’s development programs. Prior to joining Usona, senior leadership positions in the pharma and biotech industries in Switzerland and ^or ^e corporate and finance practice group at Powell Goldstein LLP in Washington, he served as Chief Operating Officer of Olatec Therapeutics where he oversaw the in the US (Merck, Sharp and Dohme, Roche and Actelion pharmaceuticals) covering DC, where she advised clients on all corporate and financing matters, including complex execution of numerous early- and late-stage clinical trials in the fields of analgesics, Product Development, R&D, and Strategic Marketing. Her extensive pharmaceutical public and private financings, mergers and acquisitions, SEC and regulatory rheumatology, immunology and cardiovascular disease. Rob holds a Master’s degree industry and biomed research and development experience covers the full spectrum compliance, and corporate governance and compliance. Before that, Ms. Hu was in Pharmacology from The Ohio State University and a Bachelor of Science degree of areas and activities from Preclinical to Clinical Development and Strategy, to counsel for a NYSE-listed financial institution and prior to that was in private law from Wake Forest University, where he graduated summa cum laude. Drug Registration and Launch, across several Therapeutic Areas. practice at Klehr, Harrison, Harvey & Branzburg, LLP and Littman & Krooks, LLP focusing on corporate transactions and compliance with corporate and securities laws. Daniel Karlin, MD, MA Carrie Liao, CPA L_ Francois Lilienthal, MD, MBA III ’ Chief Medical Officer VP, Corporate Controller & Accounting Principal Chief Commercial Officer Dan co-founded HealthMode in 2018 and served as its CEO until it was acquired by Carrie Liao, CPA is an active Certified Public Accountant in the Francois is a globally accomplished biopharmaceutical executive and a trained MindMed in 2021. Before that, he built and led Clinical, Informatics, and Regulatory state of California with over 20 years of accounting and finance physician with extensive experience leading end-to-end development and Strategy for Pfizer Digital Medicine and Innovation Research Lab. He also served as a leadership experience in public and private companies. Starting at Deloitte, commercialization of innovative medicines, driving significant growth across diversified Global Clinical Lead for psychiatric clinical assets at Pfizer. Previously, he was the her career has focused on the life science industry from early development through portfolios through product launches, life cycle management and business development, founder and Chief Medical Officer at Column Health, a leading technology-enabled commercialization and manufacturing. Recently, she has successfully supported Before joining MindMed, Francois was a Vice President at Merck’s commercial division psychiatry and addiction practice. He is a founding board member of the Digital multiple Initial Public Offering filings. She specializes in Sarbanes-Oxley Act for 14 years. He built and led a new department focused on developing the commercial Medicine Society, and a strategic advisor to multiple big pharma and digital compliance, resolution of complex accounting issues, process improvement, and strategy for multiple products across several therapeutic areas, including neurology, therapeutics companies. Dan is board Certified in Psychiatry, Addiction Medicine, and U.S. Securities and Exchange Commission interim and annual filings. psychiatry and pain. He previously drove double-digit growth of the Virology and Liver Clinical Informatics. He is an Assistant Professor of Psychiatry at Tufts University Diseases global business and oversaw global launches of innovative brands for the School of Medicine. He graduated with degrees in Neuroscience and Behavior (BA), treatment of HIV and chronic hepatitis C. and Clinical Informatics (MA) from Columbia University; and Medicine (MD) from the University of Colorado School of Medicine. MindMed Corporate Overview | April 2022 34 Scientific Advisory Board
Robert Malenka, MD, PhD Maria A Oquendo, MD, PhD Maurizio Fava, MD Chairman of the Scientific Advisory Board, Nancy Friend Pritzker Professor in Ruth Meltzer Professor and Chairman of Psychiatry at University of Pennsylvania, Psychiatrist-in-Chief of the Massachusetts General Hospital (MGH), director, Division of Psychiatry and Behavioral Sciences at Stanford University. Dr. Malenka is an Psychiatrist-in-Chief at the Hospital of the University of Pennsylvania. Dr. Clinical Research of the MGH Research Institute, executive director of the Clinical Trials elected member of the National Academy of Sciences and the National Academy Oquendo is a member of the National Academy of Medicine, one of the highest Network and Institute, (MGH), associate dean for clinical and translational research and of Medicine as well as an elected fellow of the American Academy of Arts and honors in medicine. She is Past President of the American Psychiatric Association the Slater Family Professor of Psychiatry at Harvard Medical School. Dr. Fava is a world Sciences, the American Association for the Advancement of Science, and the (APA), the International Academy of Suicide Research and the American College leader in the field of depression. He has edited eight books and authored or co-authored American College of Neuropsychopharmacology. He has served on the National of Neuropsychopharmacology (ACNP). She is President of the American more than 800 original articles published in medical journals with international circulation. Advisory Council on Drug Abuse and as a Counselor for the Society for Foundation for Suicide Prevention’s Board of Directors, Vice President of the articles which have been cited more than 80,000 times in the literature and with an h Neuroscience and the American College of Neuropsychopharmacology. He is College of International Neuropsychopharmacology and has served on the index of over 140. Dr. Fava is a world leader in the field of depression. He has edited eight known for his landmark contributions to understanding of brain plasticity National Institute of Mental Health’s Advisory Council. Dr. Oquendo is a member books and authored or co-authored more than 800 original articles published in medical mechanisms, and has extensive experience as an advisor to various of Tufts University’s Board of Trustees, serves on its Executive Committee and journals with international circulation, articles which have been cited more than 80,000 pharmaceutical and biotechnology companies. chairs Tufts’ Academic Affairs Committee. times in the literature and with an h index of over 140. Robert H. Dworkin, PhD Peter Bergethon, MD Bryan L Roth, MD, PhD Professor of Anesthesiology ond Perioperative Medicine, Neurology, and VP and Head of Quantitative & Clinical Technologies, Biogen, Inc., where he leads Professor of Pharmacology, University of North Carolina School of Medicine; Director Psychiatry, and Professor in the Center for Health + Technology, at the University the effort to transform clinical trials and humanize drug discovery by encouraging of the National Institute of Mental Health Psychoactive Drug Screening Program. Dr. of Rochester School of Medicine and Dentistry. Dr. Dworkin has spent over 35 the transition of clinical trial measures from a qualitative to a quantitative Roth has spent over 30 years studying molecular neuropharmacology. He is the years conducting clinical research on pain. He is also Director of the Analgesic, discipline. The Quantitative Medicine transformation has advanced Biogen’s Michael Hooker Distinguished Professor in the Department of Pharmacology at the Anesthetic, and Addiction Clinical Triol Translations, Innovations, Opportunities, leadership in neuroscience therapeutics and personalized medicine. Dr. Bergethon University of North Carolina s (UNC) School of Medicine. He is also the director of the and Networks (ACTTION) public-private partnership with the U.S. Food and Drug came to Biogen in 2017 from Pfizer Worldwide Research and Development where National Institute of Mental Health Psychoactive Drug Screening Program. Dr. Roth’s Administration (FDA). Dr. Dworkin received the American Pain Society’s Wilbert E. he was Vice President and Head of the Pfizer Innovation Research Lab within the research focuses on removing the hallucinogenic effects from psychedelic drugs, Fordyce Clinical Investigator Award in 2005 and John and Emma Bonica Public Early Clinical Development group. Before joining the biopharmaceutical industry in eliminating the hours long hallucinatory trips that may turn some patients away from Service Award in 2014, the American Academy of Neurology’s Mitchell B. Max 2012, Dr. Bergethon spent 30 years in acodemic medicine as a Professor at Boston psychedelic assisted treatments. He was among the first to publish in the scientific . , ., journal, Cell, the structure of how LSD binds to serotonin receptors within the brain. Award for Neuropathic Pain in 2015, and the International Association for the University and Tufts University in the Departments of Biochemistry, Neurology,.... This structure is crucial to help scientists understand why psychedelics can have Study of Pain’s John D. Loeser Award in 2020. Neurobiology & Anatomy, and Biomedical Engineering. . ,, . hallucinogenic and therapeutic effects. MindMed Corporate Overview | April 2022 35

MindMed MindMed Corporate Overview | April 2022 36