Exhibit 99.1

MindMed Discover. Develop. Deploy. www.mindmed.co Psychedelic Inspired Medicines August 2021 MMED MNMD MMQ NASDAQ NEO DE MindMed

Disclaimer Corporate Presentation | August 2021 MindMed 1 This presentation (the “Presentation”) has been prepared by Mind Medicine (MindMed) Inc. (“MindMed” or the “Company”) solely for informational purposes. None of MindMed, its affiliates or any of their respective employees, directors, officers, contractor s, advisors, members, successors, representatives or agents makes any representation or warranty as to the accuracy or completeness of any information containe d i n this Presentation and shall have no liability for any representations (expressed or implied) contained in, or for any omiss ion s from, this Presentation. This presentation shall not constitute an offer, nor a solicitation of an offer, of the sale or purchase of securities. This Presentation does not constitute an offering of securities of MindMed and under no circumstances is it to be construed as a prospectus or advertise me nt or public offering of securities. Market and Industry Data This Presentation by includes market and industry data that has been obtained from third party sources, including industry pu bli cations. MindMed believes that the industry data is accurate and that the estimates and assumptions are reasonable, but there is no assurance as to the accuracy or completeness of this data. Third party sources generally state that the information contained therein has been obtained from sou rces believed to be reliable, but there is no assurance as to the accuracy or completeness of included information. Although the data is believed to be reliable, MindMed has not independently verified any of the data from third party sources referred to in this Presentation or ascertained the u nde rlying economic assumptions relied upon by such sources. References in this Presentation to research reports or to articles a nd publications should be not construed as depicting the complete findings of the entire referenced report or article. MindMed does not make any representation as to th e a ccuracy of such information Cautionary Note on Forward - Looking Information This Presentation contains “forward - looking statements” and “forward - looking information” within the meaning of applicable secur ities laws (collectively, “forward - looking information”) with respect to MindMed and its subsidiaries. Statements in this Presen tation that are forward - looking information are based on currently available competitive, financial, and economic data and operating plans as of the date of thi s Presentation but are subject to various risks and uncertainties concerning the specific factors disclosed herein. Often, bu t n ot always, forward - looking information can be identified by the use of words such as “plans”, “expects”, “is expected”, “budget”, “scheduled”, “estimates”, “forecasts”, “i nte nds”, “anticipates”, will”, “projects”, or “believes” or variations (including negative variations) of such words and phrases , o r statements that certain actions, events, results or conditions “may”, “could”, “would”, “might” or “will” be taken, occur or be achieved. Except for sta tements of historical fact, information contained herein constitutes forward - looking information, including, but not limited to: statements pertaining to the development and commercialization of any medicine or treatment, or the efficacy of either of the foregoing, the likelihood of su ccess of any clinical trials or of obtaining FDA or other regulatory approvals, the likelihood of obtaining patents or the ef fic acy of such patents once granted, and the potential for the markets that MindMed is anticipating to access. Forward - looking information is not a guarantee of future performance and is based upon a number of estimates and assumptions of management at the date the statements are made including among other things assumptions about: MindMed’s ability to raise cap ita l to complete its plans and fund its studies, the medical and commercial viability of the contemplated medicines and treatments being developed, and the abili ty of MindMed to raise additional capital in the future as MindMed continues to develop its products. While MindMed considers th ese assumptions to be reasonable, the assumptions are inherently subject to significant business, social, economic, political, regulatory, competitive and other ri sks and uncertainties, contingencies and other factors that could cause actual performance, achievements, actions, events, result s or conditions to be materially different from those projected in the forward - looking information. These include the Company’s history of negative cash flows; limited operatin g history; incurrence of future losses; availability of additional capital; lack of revenue; compliance with laws and regulat ion s; difficulty associated with research and development; risks associated with clinical trials or studies; heightened regulatory scrutiny; early stage product developmen t; clinical trial risks; regulatory approval processes; novelty of the psychedelic inspired medicines industry; as well as those ri sk factors discussed or referred to under the headings “Risk Factors” in the Company’s final base shelf prospectus dated April 9, 2021 filed with the securities regulatory au thorities in each of the provinces and territories of Canada and the Company’s annual information form for the year ended Dec emb er 31, 2020 filed with the securities regulatory authorities in all provinces and territories of Canada and available under the Company’s profile on SEDAR at www.s eda r.com and as described in the Company’s U.S. registration statement on Form F - 10 declared effective by the United States Securit ies and Exchange Commission (the “SEC”) on April 12, 2021 and filed with the SEC on EDGAR at www.sec.gov. Many assumptions are based on factors and events tha t a re not within the control of MindMed and there is no assurance they will prove to be correct. The United States federal government regulates drugs through the Controlled Substances Act. The Company works with a non - halluci nogenic synthetic derivative of the psychedelic substance ibogaine, known as “18 - MC”, which is a synthetic organic molecule desi gned around a common coronaridine chemical backbone. 18 - MC is not a Schedule I substance in the United States and the Company does not foresee it bec oming a Schedule I substance due to its non - hallucinogenic properties. While the Company is focused on programs using psychedeli c inspired compounds and classic psychedelics, the Company does not have any direct or indirect involvement with the illegal selling, production or distributi on of any substances in the jurisdictions in which it operates. The Company is a neuro - pharmaceutical drug development company and does not deal with psychedelic substances except within laboratory and clinical trial settings conducted within approved regulatory frameworks. The Company’ s p roducts will not be commercialized prior to applicable regulatory approval, which will only be granted if clinical evidence o f s afety and efficacy for the intended uses is successfully developed. Although MindMed has attempted to identify important factors that could cause actual results, performance or achievements to dif fer materially from those contained in the forward - looking information, there can be other factors that cause results, performan ce or achievements not to be as anticipated, estimated or intended, including, but not limited to: MindMed not being able to obtain the necessary FDA and oth er approvals, inconclusive or negative results from clinical trials, MindMed electing to not proceed with any of the medicines o r t reatments discussed herein, and MindMed not being able to build production capacity should its trials be successful. To the extent any forward - looking information conta ins forecasts or financial outlooks, such information is being provided solely to enable a reader to assess MindMed’s financi al condition and its operational history and experience in the pharmaceutical industry. Readers are cautioned that this information may be not appropriate for any other p urp ose, including investment decisions. Such information, as with forward - looking information generally, is, without limitation, ba sed on the assumptions and subject to the risks and other cautionary statements set out above. The actual results achieved will vary from the forecast or financial out loo k results and the variations may be material. No representation or warranty of any kind is or can be made with respect to the ac curacy or completeness of, and no representation or warranty should be inferred from, our projections or the assumptions underlying them. There can be no assurance that such information will prove to be accurate or that management’s expectations or estimates of f utu re developments, circumstances or results will materialize. As a result of these risks and uncertainties, the results or even ts predicted in this forward - looking information may differ materially from actual results or events. Because of the risks, uncertainties and assumptions contained herein, re ade rs should not read forward - looking information as guarantees of future performance or results. Nothing in this presentation is, or should be relied upon as, a promise or representation as to the future. All amounts are in USD unless otherwise noted. Accordingly, readers should not place undue r eli ance on forward - looking information. The forward - looking information in this Presentation is made as of the date of this Present ation. MindMed disclaims any intention or obligation to update or revise such information, except as required by applicable law, and MindMed does not assume any liabil ity for disclosure relating to any other company mentioned herein. Investors are cautioned not to unduly rely on this forward - look ing information and are encouraged to read MindMed’s continuous disclosure documents, including its current annual information form, as well as its audited annual con solidated financial statements which are available on SEDAR at www.sedar.com and on EDGAR at www.sec.gov/edgar. MindMed’s securities have not been approved or disapproved by the SEC or by any state, provincial or other securities regulat ory authority, nor has the SEC or any state, provincial or other securities regulatory authority passed on the accuracy or adequa cy of this Presentation. Any representation to the contrary is a criminal offense.

Leadership: A Combination of Drug Developers & Technologists Corporate Presentation | August 2021 MindMed 2 Daniel Karlin, MD, MA Bradford Cross Miri Halperin Wernli, PhD Robert Barrow Chief Medical Officer Chief Technology Officer Executive President & Board Director Chief Executive Officer Dan previously co - founded HealthMode in 2018 and served as CEO until its acquisition by MindMed. Before that, he built and led Clinical, Informatics, and Regulatory Strategy for Pfizer’s Digital Medicine and Innovation Research Lab. He also served as Global Clinical Lead for psychiatry clinical compounds at Pfizer. Previously, he was the founder and Chief Medical Officer at Column Health, a leading technology - enabled psychiatry and addiction practice. He is a strategic advisor to multiple big pharma, and digital therapeutic companies. Dan is board Certified in Psychiatry, Addiction Medicine, and Clinical Informatics. He is an Asst. Prof. of Psychiatry at Tufts University School of Medicine. He graduated with degrees in Neuroscience and Behavior (BA), and Clinical Informatics (MA), Columbia University; Medicine (MD), University of Colorado School of Medicine. Miri co - founded Creso Pharma, a cannabis company, and listed the company on the Australian Stock exchange (ASX) in October 2016. Prior to founding Creso Pharma Dr. Halperin Wernli worked in clinical psychiatry in Swiss academic hospital settings and then held various global senior leadership positions in the pharma and biotech industries in Switzerland and in the US (Merck, Sharp and Dohme, Roche and Actelion pharmaceuticals) covering Product Development, R&D, and Strategic Marketing. Her extensive pharmaceutical industry and biomed research and development experience covers the full spectrum of areas and activities from Preclinical to Clinical Development and Strategy, to Drug Registration and Launch, across several Therapeutic Areas. Rob is an accomplished pharmaceutical executive and clinical pharmacologist with over a decade of experience leading drug development programs in a variety of disease areas. Mr. Barrow previously served as Director of Drug Development & Discovery at Usona Institute, where he oversaw preclinical, clinical and regulatory development efforts for all of Usona’s development programs. Prior to joining Usona, he served as Chief Operating Officer of Olatec Therapeutics where he oversaw the execution of numerous early - and late - stage clinical trials in the fields of analgesics, rheumatology, immunology and cardiovascular disease. Rob holds a Master’s degree in Pharmacology from The Ohio State University and a Bachelor of Science degree from Wake Forest University, where he graduated summa cum laude. Bradford previously co - founded HealthMode in 2018 and served as its CTO until its acquisition by MindMed. He is a career entrepreneur and investor with 15+ years at the intersection of AI and startups, and finance. Founded Prismatic in 2012, which powered part of LinkedIn’s news feed as of 2015. Machine learning for personalization and content classification. Founded DCVC in 2011, which has grown into a $2B+ leading deep tech VC investing heavily at the intersection of computation and bio and spinning up dedicated DCVC bio fund. Founded Flightcaster in 2009, first AI Startup in YCombinator. He previously worked in distributed systems at Google 2007 - 2009. Brad earned degrees in Computer Science and Finance at Virginia Tech, and Mathematics at Berkeley. Dave Guebert Chief Financial Officer Dave Guebert is a CPA, qualified in both Alberta and Pennsylvania, and a Member of the Institute of Corporate Directors. He started his career in 1979 at Deloitte where he qualified for his CPA designations. He went on to serve as the Controller for the XV Olympic Winter Games from 1986 to 1988. Since then has taken on increasing senior roles, acting as Chief Financial Officer for a number of public and private companies, primarily in the technology industry. He currently sits as a board member and Audit Committee Chair for Legend Power Systems (TSXV: LPS), RMMI Inc. (CSE: RMMI) and Quisitive Technology Solutions, Inc. (TSXV: QUIS). From 2010 to 2017, he was board member and Audit Committee Chair of Merus Labs International Inc. (TSX: MSL; NSDQ: MSLI), a specialty pharmaceutical company. Donald Gehlert, PhD Chief Scientific Officer Don has extensive experience in drug discovery and expertise in key functional areas of exploratory development and disease biology. During his career at Lilly, Don led or participated in teams that introduced 19 molecules into the Lilly pipeline including both small and large molecule therapies. He also participated on Phase I and Phase II clinical development teams that designed and delivered translational proof of concept studies in the areas of ADHD, obesity, AUD, depression, pain and migraine. He is a co - author on 182 publications and a co - inventor on 15 issued and pending patents.
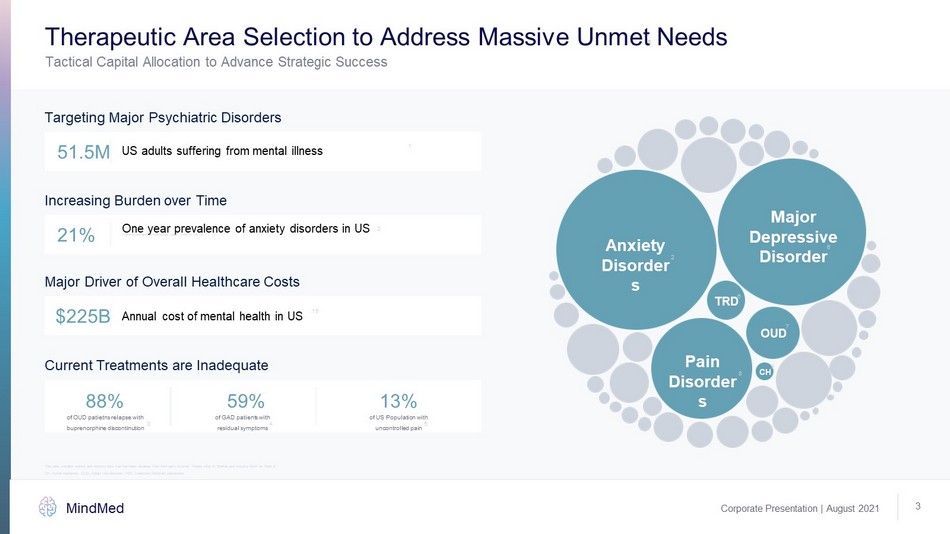
Corporate Presentation | August 2021 MindMed 3 8 Tactical Capital Allocation to Advance Strategic Success Therapeutic Area Selection to Address Massive Unmet Needs Targeting Major Psychiatric Disorders Increasing Burden over Time Anxiety Disorders Major Depressive Disorder Pain Disorders OUD TRD CH 51.5 M 21% US adults suffering from mental illness One year prevalence of anxiety disorders in US Statistic pertains to individuals aged 18 - 64 in the US Major Driver of Overall Healthcare Costs $225B Annual cost of mental health in US Current Treatments are Inadequate 88% 59% 13% of OUD patietns relapse with buprenorphine discontinution of GAD patients with residual symptoms CH: cluster headache; OUD: Opioid Use Disorder; TRD: Treatment Resistant Depression 1 2 2 8 9 6 6 7 15 3 4 5 of US Population with uncontrolled pain This slide contains market and industry data that has been obtained from third party sources. Please refer to “Market and Ind ust ry Data” on Slide 2
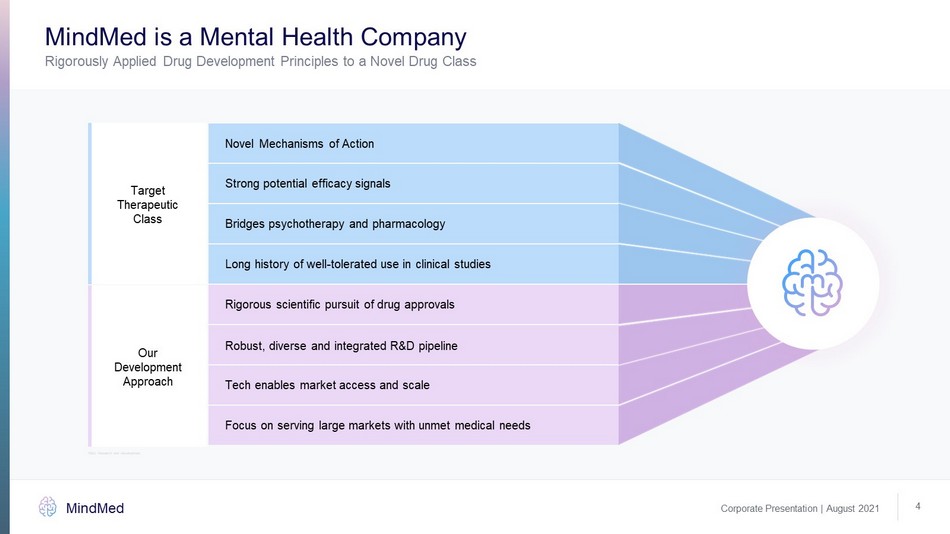
MindMed is a Mental Health Company Corporate Presentation | August 2021 MindMed Rigorously Applied Drug Development Principles to a Novel Drug Class 4 Novel Mechanisms of Action Strong potential efficacy signals Bridges psychotherapy and pharmacology Long history of well - tolerated use in clinical studies Rigorous scientific pursuit of drug approvals Robust, diverse and integrated R&D pipeline Tech enables market access and scale Focus on serving large markets with unmet medical needs Our Development Approach Target Therapeutic Class R&D: Research and Development
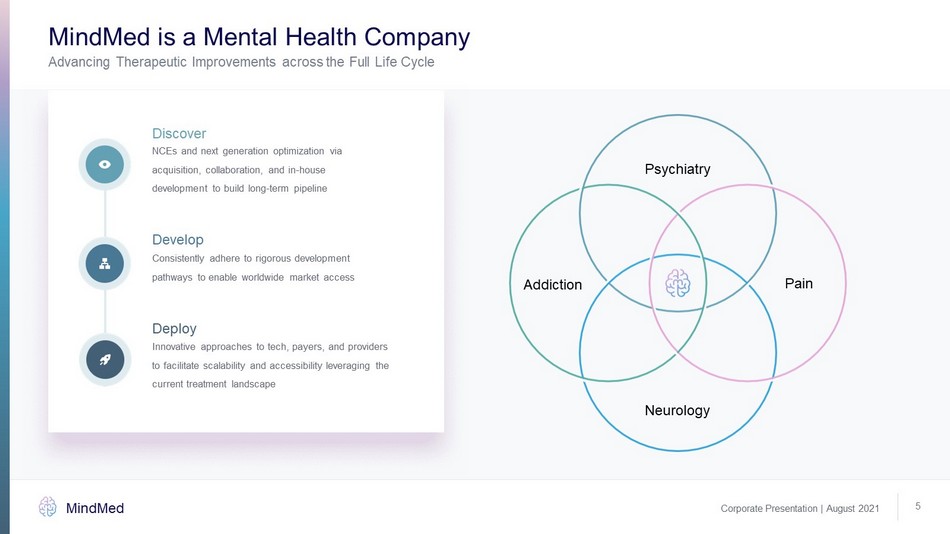
Corporate Presentation | August 2021 MindMed 5 Deploy Innovative approaches to tech, payers, and providers to facilitate scalability and accessibility leveraging the current treatment landscape Develop Consistently adhere to rigorous development pathways to enable worldwide market access Discover NCEs and next generation optimization via acquisition, collaboration, and in - house development to build long - term pipeline MindMed is a Mental Health Company Advancing Therapeutic Improvements across the Full Life Cycle Psychiatry Pain Neurology Addiction
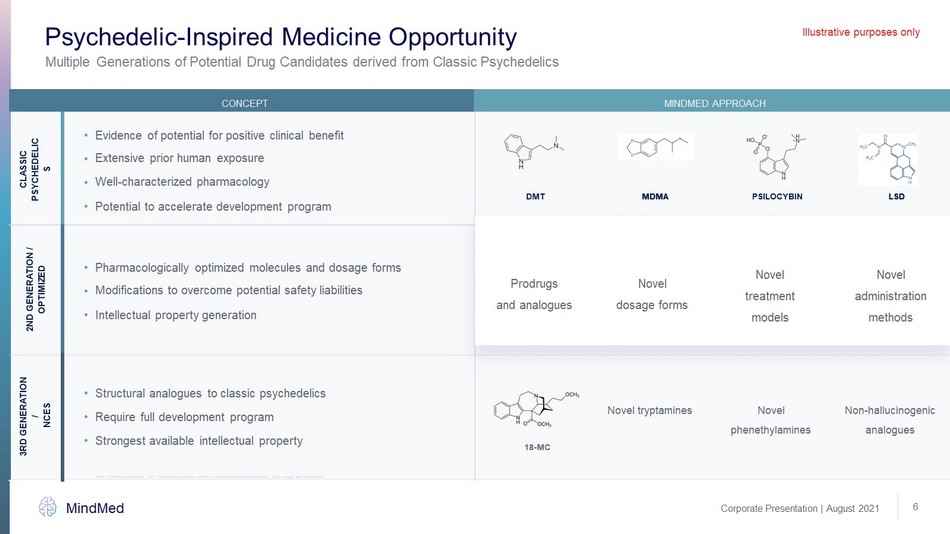
Corporate Presentation | August 2021 MindMed 6 Multiple Generations of Potential Drug Candidates derived from Classic Psychedelics Psychedelic - Inspired Medicine Opportunity Illustrative purposes only Evidence of potential for positive clinical benefit • Novel tryptamines Novel phenethylamines Non - hallucinogenic analogues Pharmacologically optimized molecules and dosage forms • Extensive prior human exposure • Well - characterized pharmacology • Modifications to overcome potential safety liabilities • Potential to accelerate development program • Intellectual property generation • CLASSIC PSYCHEDELICS 2ND GENERATION / OPTIMIZED 3RD GENERATION / NCES CONCEPT MINDMED APPROACH Structural analogues to classic psychedelics • Strongest available intellectual property • Require full development program • Novel treatment models Novel administration methods Novel dosage forms Prodrugs and analogues 18 - MC: 18 - Methoxycoronaridine, DMT: N,N - Dimethyltryptamine, MDMA: 3,4 - Methylenedioxymethamphetamine, LSD: Lysergic acid diethy lamide MDMA LSD DMT PSILOCYBIN 18 - MC MDMA LSD

Advancing the Field with Strong IP & Strategic Competitive Moats Corporate Presentation | August 2021 MindMed 7 Protecting Innovation and Market Potential Our Patent Positions are Extensive and Diverse 45+ patent applications filed 45+ molecules covered 10+ patent applications covering LSD 3+ patent applications covering 18 - MC Strategic Approach to Maximize Market Protection Composition of matter Proprietary methods of manufacturing Combination therapies NCEs/psychedelic analogues AI and ML algorithms Unexplored indications Optimized formulations with unique PK Innovative dosing protocols Physiochemical attributes 30+ NCEs covered 18 - MC: 18 - Methoxycoronaridine, AI: artificial intelligence, IP: intellectual property, LSD: lysergic acid diethylamide, ML: mach ine learning, NCE: new chemical entity, PK: pharmacokinetic

Corporate Presentation | August 2021 MindMed 8 Science & Background The Case for Psychedelic - Inspired Therapies
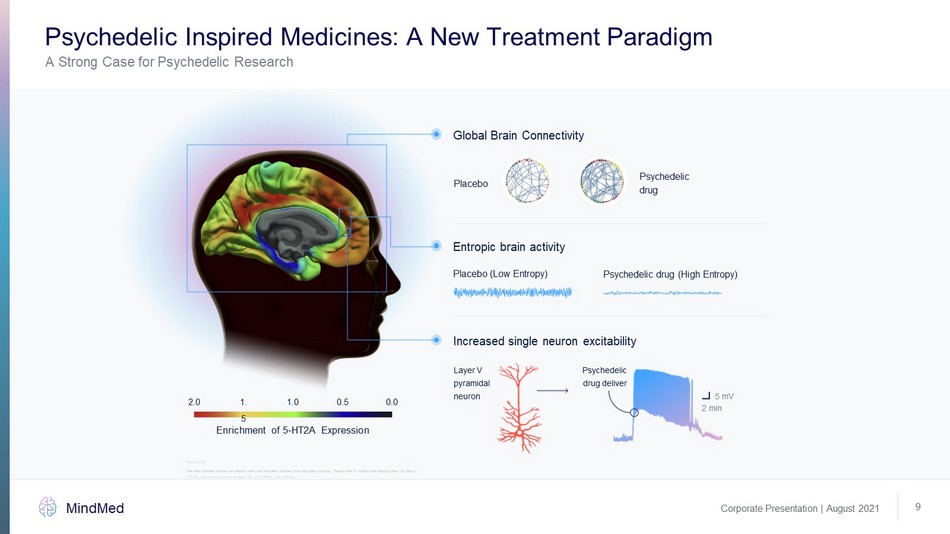
Corporate Presentation | August 2021 MindMed A Strong Case for Psychedelic Research Psychedelic Inspired Medicines: A New Treatment Paradigm 9 Global Brain Connectivity Enrichment of 5 - HT2A Expression 2.0 1.5 1.0 0.5 0.0 Placebo Increased single neuron excitability Layer V pyramidal neuron Psychedelic drug deliver 5 mV 2 min Entropic brain activity Placebo (Low Entropy) Psychedelic drug (High Entropy) Psychedelic drug Source: [10] 5 - HT2A: 5 - hydroxytryptamine receptor 2A, mV: millivolt, min: minutes This slide contains market and industry data that has been obtained from third party sources. Please refer to “Market and Ind ust ry Data” on Slide 2 This slide contains market and industry data that has been obtained from third party sources. Please refer to “Market and Ind ust ry Data” on Slide 2
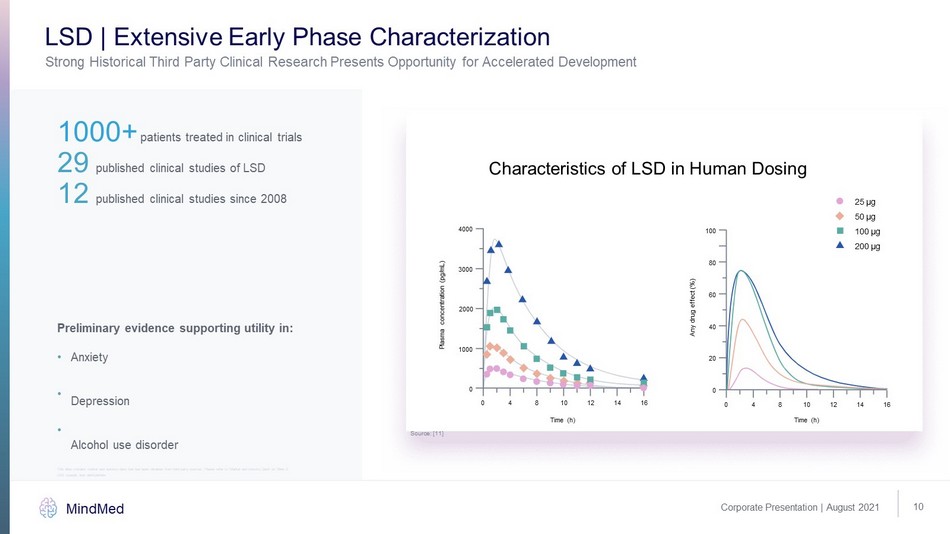
This slide contains market and industry data that has been obtained from third party sources. Please refer to “Market and Ind ust ry Data” on Slide 2 10 Corporate Presentation | August 2021 MindMed Strong Historical Third Party Clinical Research Presents Opportunity for Accelerated Development LSD | Extensive Early Phase Characterization 1000+ patients treated in clinical trials 29 published clinical studies of LSD 12 published clinical studies since 2008 Preliminary evidence supporting utility in: Anxiety Depression Alcohol use disorder 4000 25 μg 50 μg 100 μg 200 μg 3000 2000 Plasma concentration (pg/mL) Time (h) 1000 0 0 4 8 10 12 14 16 100 80 60 40 Any drug effect (%) 20 0 0 4 8 10 12 14 16 Time (h) Characteristics of LSD in Human Dosing Source: [11] • • • LSD: lysergic acid diethylamide
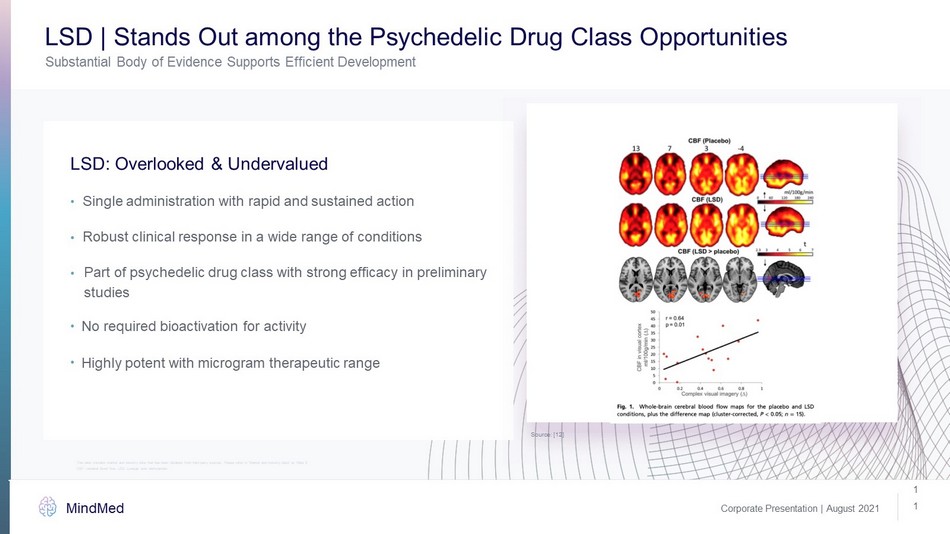
Corporate Presentation | August 2021 MindMed 1 1 Substantial Body of Evidence Supports Efficient Development LSD | Stands Out among the Psychedelic Drug Class Opportunities LSD: Overlooked & Undervalued Single administration with rapid and sustained action • Robust clinical response in a wide range of conditions • Part of psychedelic drug class with strong efficacy in preliminary studies • No required bioactivation for activity • Highly potent with microgram therapeutic range • Source: [12] CBF: cerebral blood flow, LSD: Lysergic acid diethylamide This slide contains market and industry data that has been obtained from third party sources. Please refer to “Market and Ind ust ry Data” on Slide 2
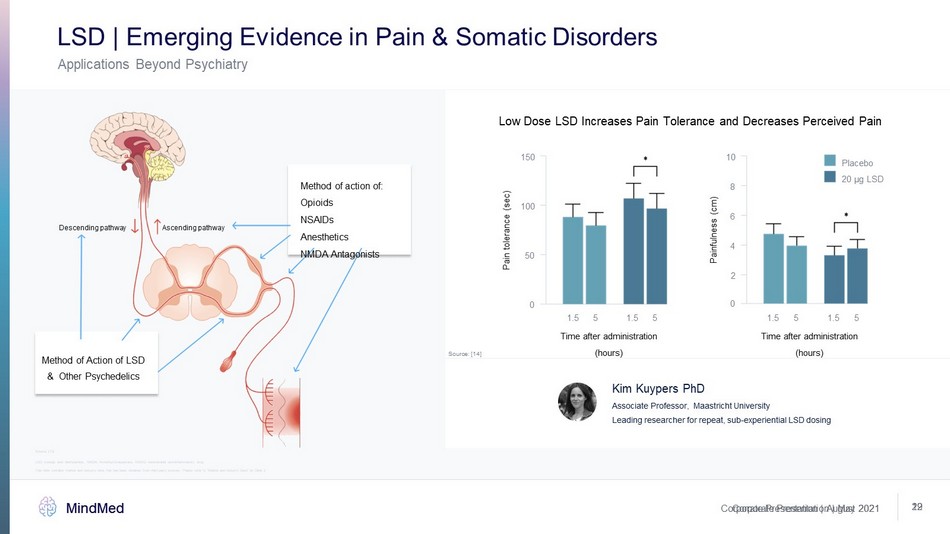
Corporate Presentation | May 2021 MindMed 29 Corporate Presentation | August 2021 MindMed 12 Applications Beyond Psychiatry LSD | Emerging Evidence in Pain & Somatic Disorders Low Dose LSD Increases Pain Tolerance and Decreases Perceived Pain Source: [14] Source: [13] Time after administration (hours) Pain tolerance (sec) 1.5 0 50 100 150 1.5 5 5 Painfulness (cm) 1.5 1.5 5 0 2 4 6 8 10 5 Time after administration (hours) Placebo 20 μg LSD Method of action of: Opioids NSAIDs Anesthetics NMDA Antagonists Ascending pathway Descending pathway Method of Action of LSD & Other Psychedelics Kim Kuypers PhD Associate Professor, Maastricht University Leading researcher for repeat, sub - experiential LSD dosing LSD: lysergic acid diethylamide, NMDA: N - methyl - D - aspartate, NSAID: nonsteroidal anti - inflammatory drug This slide contains market and industry data that has been obtained from third party sources. Please refer to “Market and Ind ust ry Data” on Slide 2
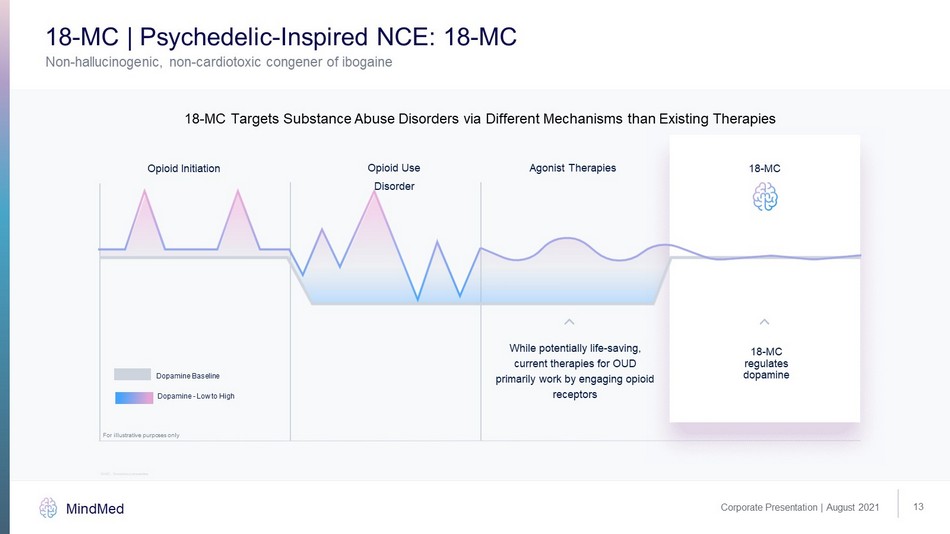
13 Corporate Presentation | August 2021 MindMed Non - hallucinogenic, non - cardiotoxic congener of ibogaine 18 - MC | Psychedelic - Inspired NCE: 18 - MC 18 - MC regulates dopamine While potentially life - saving, current therapies for OUD primarily work by engaging opioid receptors Dopamine - Low to High Dopamine Baseline Opioid Initiation Opioid Use Disorder Agonist Therapies 18 - MC 18 - MC Targets Substance Abuse Disorders via Different Mechanisms than Existing Therapies For illustrative purposes only 18 - MC: 18 - methoxycoronaridine
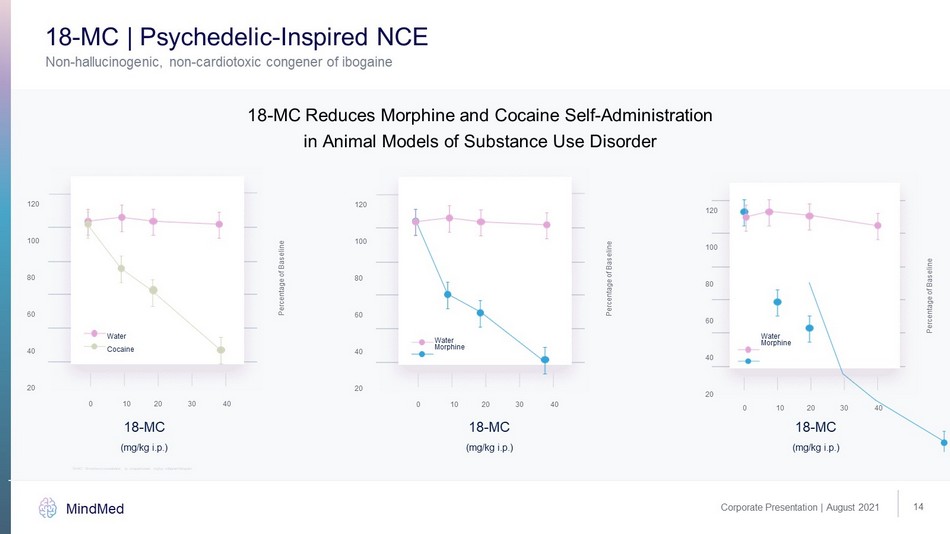
14 Corporate Presentation | August 2021 MindMed (mg/kg i.p.) 120 100 80 60 40 20 0 10 20 30 40 Percentage of Baseline 18 - MC Water Cocaine (mg/kg i.p.) 120 100 80 60 40 20 0 10 20 30 40 Percentage of Baseline 18 - MC Water Morphine Non - hallucinogenic, non - cardiotoxic congener of ibogaine 18 - MC | Psychedelic - Inspired NCE 18 - MC Reduces Morphine and Cocaine Self - Administration in Animal Models of Substance Use Disorder (mg/kg i.p.) 18 - MC 18 - MC: 18 - methoxycoronaridine, ip: ntraperitoneal, mg/kg: milligram/kilogram 120 100 80 60 40 20 0 10 20 30 40 Percentage of Baseline Water Morphine

Our Division For Concept Testing of NCEs and Classic Psychedelics Corporate Presentation | August 2021 MindMed 15 Develop & Discover
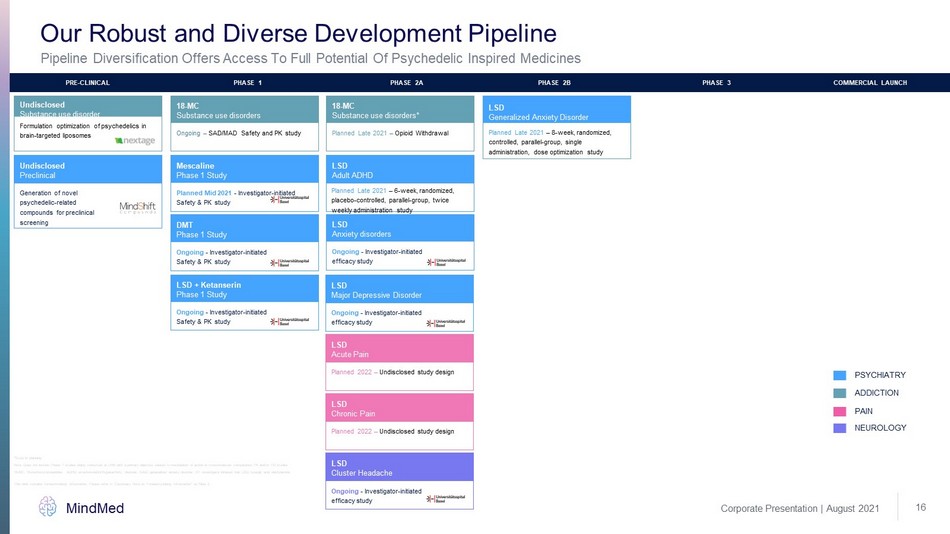
Corporate Presentation | August 2021 MindMed *Study in planning Note: Does not include Phase 1 studies being conducted at UHB with a primary objective related to mechanism of action or cros s - m olecule comparative PK and/or PD studies Pipeline Diversification Offers Access To Full Potential Of Psychedelic Inspired Medicines Our Robust and Diverse Development Pipeline 16 Planned Late 2021 – Opioid Withdrawal 18 - MC Substance use disorders* Ongoing – SAD/MAD Safety and PK study 18 - MC Substance use disorders Formulation optimization of psychedelics in brain - targeted liposomes Undisclosed Substance use disorder Planned Late 2021 – 8 - week, randomized, controlled, parallel - group, single administration, dose optimization study LSD Generalized Anxiety Disorder Planned Mid 2021 - Investigator - initiated Safety & PK study Mescaline Phase 1 Study Ongoing - Investigator - initiated Safety & PK study DMT Phase 1 Study Ongoing - Investigator - initiated Safety & PK study LSD + Ketanserin Phase 1 Study Planned 2022 – Undisclosed study design LSD Acute Pain Planned 2022 – Undisclosed study design LSD Chronic Pain LSD Cluster Headache ADDICTION PAIN NEUROLOGY PSYCHIATRY LSD Major Depressive Disorder PRE - CLINICAL PHASE 1 PHASE 2A PHASE 2B PHASE 3 COMMERCIAL LAUNCH Generation of novel psychedelic - related compounds for preclinical screening Ongoing - Investigator - initiated efficacy study Undisclosed Preclinical Ongoing - Investigator - initiated efficacy study LSD Anxiety disorders Ongoing - Investigator - initiated efficacy study Planned Late 2021 – 6 - week, randomized, placebo - controlled, parallel - group, twice weekly administration study LSD Adult ADHD 18 - MC: 18 - methoxycoronaridine, ADHD: attention - deficit/hyperactivity disorder, GAD: generalized anxiety disorder, IIT: investiga tor initiated trial, LSD: lysergic acid diethylamide This slide contains forward - looking information. Please refer to “Cautionary Note on Forward - Looking Information” on Slide 2.
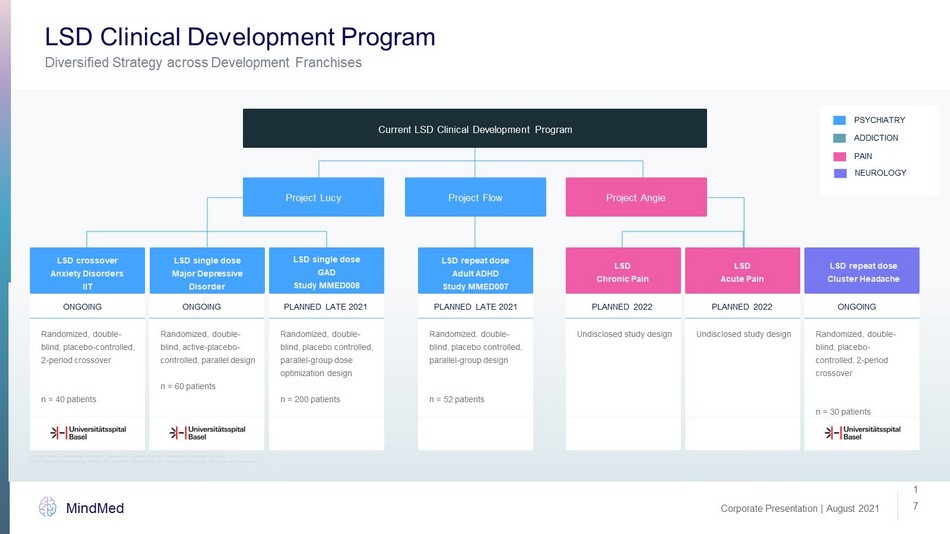
Corporate Presentation | August 2021 MindMed 1 7 Diversified Strategy across Development Franchises LSD Clinical Development Program Randomized, double - blind, placebo - controlled, 2 - period crossover n = 40 patients ONGOING Randomized, double - blind, active - placebo - controlled, parallel design n = 60 patients ONGOING Randomized, double - blind, placebo controlled, parallel - group dose optimization design n = 200 patients PLANNED LATE 2021 Randomized, double - blind, placebo controlled, parallel - group design n = 52 patients PLANNED LATE 2021 Undisclosed study design PLANNED 2022 Undisclosed study design PLANNED 2022 Randomized, double - blind, placebo - controlled, 2 - period crossover n = 30 patients ONGOING Current LSD Clinical Development Program Project Angie Project Flow Project Lucy LSD single dose Major Depressive Disorder IIT LSD repeat dose Adult ADHD Study MMED007 LSD Chronic Pain LSD Acute Pain LSD repeat dose Cluster Headache LSD single dose GAD Study MMED008 ADDICTION PAIN NEUROLOGY PSYCHIATRY LSD crossover Anxiety Disorders IIT ADHD: attention - deficit/hyperactivity disorder, GAD: generalized anxiety disorder, IIT: investigator initiated study, LSD: lyser gic acid diethylamide, This slide contains forward - looking information. Please refer to “Cautionary Note on Forward - Looking Information” on Slide 2.
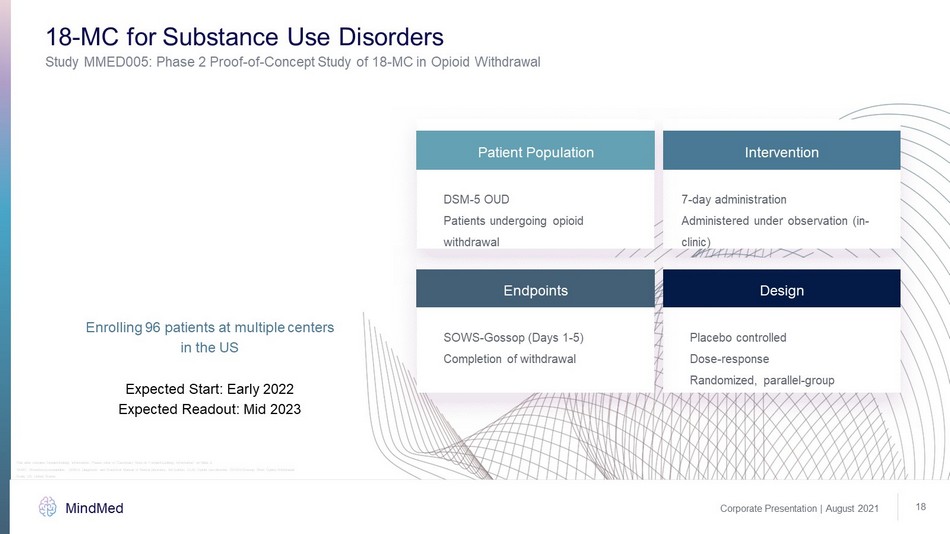
Enrolling 96 patients at multiple centers in the US Expected Start: Early 2022 Expected Readout: Mid 2023 Study MMED005: Phase 2 Proof - of - Concept Study of 18 - MC in Opioid Withdrawal 18 - MC for Substance Use Disorders Endpoints Design DSM - 5 OUD Patients undergoing opioid withdrawal SOWS - Gossop (Days 1 - 5) Completion of withdrawal Placebo controlled Dose - response Randomized, parallel - group 7 - day administration Administered under observation (in - clinic) Patient Population Intervention Corporate Presentation | August 2021 MindMed 18 18 - MC: 18 - methoxycoronaridine, DSM - 5: Diagnostic and Statistical Manual of Mental Disorders, 5th Edition, OUD: Opioid use disord er, SOWS - Gossop: Short Opiate Withdrawal Scale, US: United States This slide contains forward - looking information. Please refer to “Cautionary Note on Forward - Looking Information” on Slide 2.
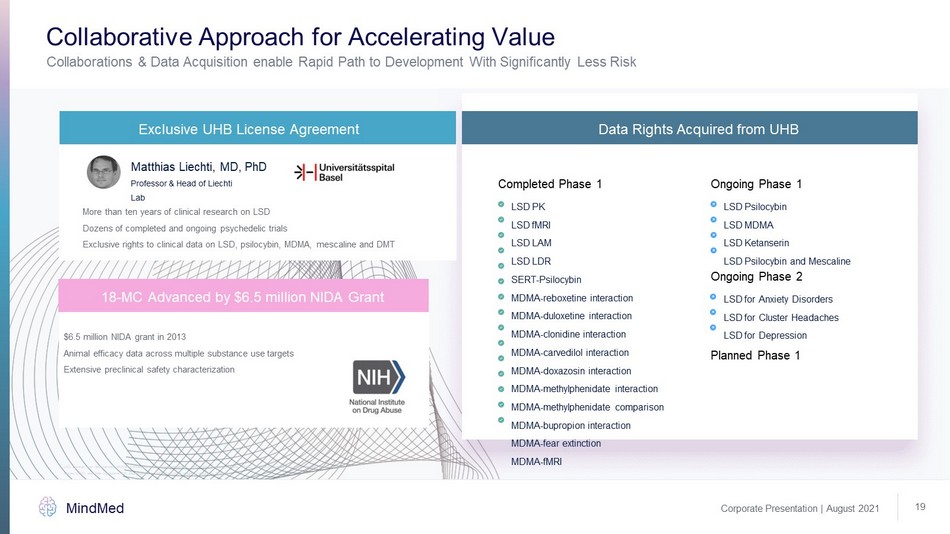
Corporate Presentation | August 2021 MindMed 19 Collaborations & Data Acquisition enable Rapid Path to Development With Significantly Less Risk Collaborative Approach for Accelerating Value Completed Phase 1 Ongoing Phase 1 Ongoing Phase 2 Planned Phase 1 LSD for Anxiety Disorders LSD for Cluster Headaches LSD for Depression LSD Psilocybin LSD MDMA LSD Ketanserin LSD Psilocybin and Mescaline Exclusive UHB License Agreement More than ten years of clinical research on LSD Dozens of completed and ongoing psychedelic trials Exclusive rights to clinical data on LSD, psilocybin, MDMA, mescaline and DMT Matthias Liechti, MD, PhD Professor & Head of Liechti Lab 18 - MC Advanced by $6.5 million NIDA Grant Data Rights Acquired from UHB $6.5 million NIDA grant in 2013 Animal efficacy data across multiple substance use targets Extensive preclinical safety characterization LSD: Lysergic acid diethylamide, MDMA: 3,4 - Methylenedioxymethamphetamine, DMT: N,N - Dimethyltryptamine, 18 - MC: 18 - Methoxycorona ridine, NIDA: National Institute on Drug Abuse, SERT: serotonin transporter, PK: pharmokinetics, FMRI: Functional magnetic resonance imaging, , LSD PK LSD fMRI LSD LAM LSD LDR SERT - Psilocybin MDMA - reboxetine interaction MDMA - duloxetine interaction MDMA - clonidine interaction MDMA - carvedilol interaction MDMA - doxazosin interaction MDMA - methylphenidate interaction MDMA - methylphenidate comparison MDMA - bupropion interaction MDMA - fear extinction MDMA - fMRI

Robust pipeline driven by collaborative arrangements R&D Pipeline Corporate Presentation | August 2021 MindMed 20 IP: intellectual property, R&D: research and development • • • Exclusive rights to completed and ongoing clinical study data Execution of Phase 1/2 studies on known and novel psychedelics Intended to enable efficient de - risking of new assets • • • Portfolio of psychedelic - inspired NCEs Screening and lead optimization program ongoing Generates multiple patent filings • • Exclusive collaborative R&D agreement covering psychedelics Application of liposome technology to optimize delivery
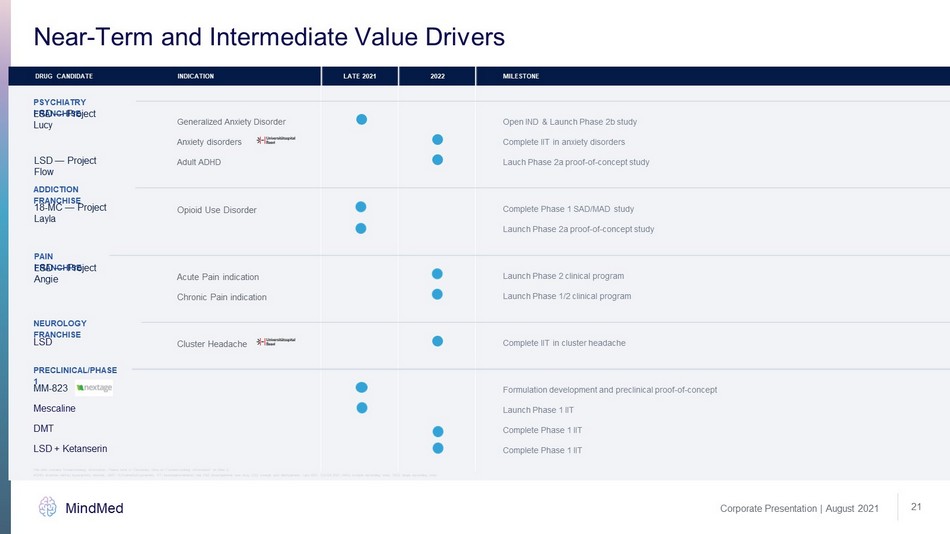
Corporate Presentation | August 2021 MindMed 21 Near - Term and Intermediate Value Drivers DRUG CANDIDATE INDICATION LATE 2021 2022 MILESTONE ADDICTION FRANCHISE 18 - MC — Project Layla Opioid Use Disorder Complete Phase 1 SAD/MAD study Launch Phase 2a proof - of - concept study PSYCHIATRY FRANCHISE Anxiety disorders Complete IIT in anxiety disorders LSD — Project Lucy Generalized Anxiety Disorder Open IND & Launch Phase 2b study LSD — Project Flow Adult ADHD Lauch Phase 2a proof - of - concept study PRECLINICAL/PHASE 1 MM - 823 Formulation development and preclinical proof - of - concept Mescaline Launch Phase 1 IIT DMT Complete Phase 1 IIT LSD + Ketanserin Complete Phase 1 IIT PAIN FRANCHISE LSD — Project Angie Acute Pain indication Launch Phase 2 clinical program Chronic Pain indication Launch Phase 1/2 clinical program NEUROLOGY FRANCHISE LSD Cluster Headache Complete IIT in cluster headache ADHD: attention deficity hyperactivity disorder, DMT: N,N - dimethyltryptamine, IIT: investigator - initiated trial, IND: investigat ional new drug, LSD: lysergic acid diethylamide, Late 2021: Q3 - Q4 2021; MAD: multiple ascending dose, SAD: single ascending dose This slide contains forward - looking information. Please refer to “Cautionary Note on Forward - Looking Information” on Slide 2.

15 Corporate Presentation | August 2021 MindMed 22 Deploy Our Division To Enable Scalability & Accessibility of Our Drug Programs

Scalable Delivery Platform to Enable Leveraging of Current Ecosystem Donating $5 million over a 5 - year period Establish the gold standard of training programs to educate healthcare providers and psychedelic researchers Use digital medicine technology to promote outcomes and engagement at all phases of the development, deployment, and clinical lifecycle Build proprietary applications to increase patient engagement, enhance provider experience, and protect market share Corporate Presentation | August 2021 MindMed 23 LSD: lysergic acid diethylamide This slide contains forward - looking information. Please refer to “Cautionary Note on Forward - Looking Information” on Slide 2. Building The Psychiatric Training Infrastructure Digital Medicine Commercial Principles for Scalability & Accessibility Innovative Medical Approaches Driving adoption of our drugs through enhancement of the existing psychiatric and psychotherapeutic infrastructure LSD neutralizer technology Value - based reimbursement models

Scalable Delivery Platform to Enable Adoption Leveraging the Current Ecosystem MindMed 24 Corporate Presentation | August 2021 Note: application images are for illustrative purposes only. Digital Medicine: Multiple components of the MindMed platform Early Engagement & Education Treatment Session Longitudinal Patient Engagement Patient education, engagement, preparation and assistance Deep digital diagnosis allows greater granularity to complement DSM diagnoses Support for treatment selection: modality dose, and timing In - session monitoring for safety, efficacy, and additional interventions Clinician decision support for drug and non - drug therapeutic sessions Predictive models linking interventions and treatment outcomes Real world monitoring of trends for relapse prediction and re - treatment decisions Engagement in health maintenance behaviors AI models to inform psychotherapeutic intervention DSM: Diagnostic and Statistical Manual of Mental Disorders AI: artificial intelligence This slide contains forward - looking information. Please refer to “Cautionary Note on Forward - Looking Information” on Slide 2.
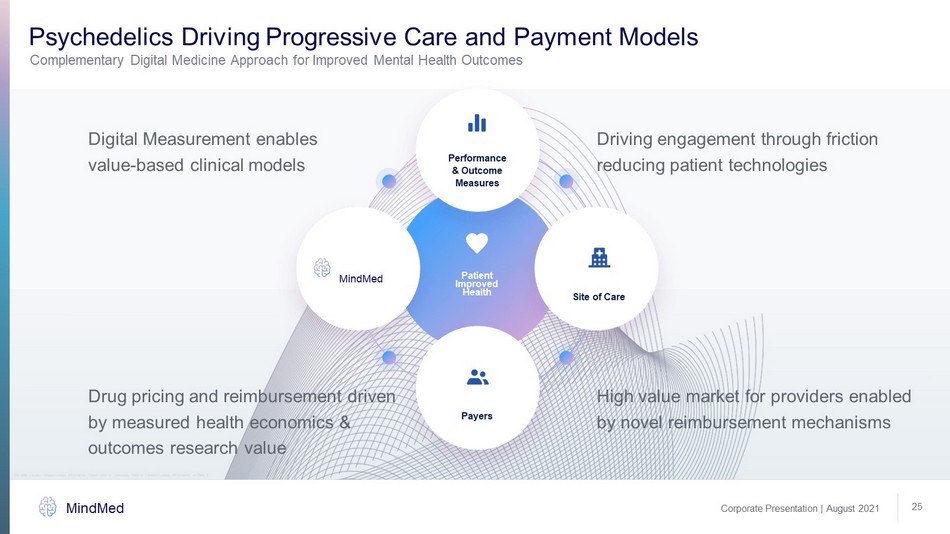
Corporate Presentation | August 2021 MindMed 25 Psychedelics Driving Progressive Care and Payment Models Complementary Digital Medicine Approach for Improved Mental Health Outcomes Digital Measurement enables value - based clinical models Driving engagement through friction reducing patient technologies High value market for providers enabled by novel reimbursement mechanisms Drug pricing and reimbursement driven by measured health economics & outcomes research value Site of Care Patient Improved Health MindMed Payers Performance & Outcome Measures This slide contains forward - looking information. Please refer to “Cautionary Note on Forward - Looking Information” on Slide 2.
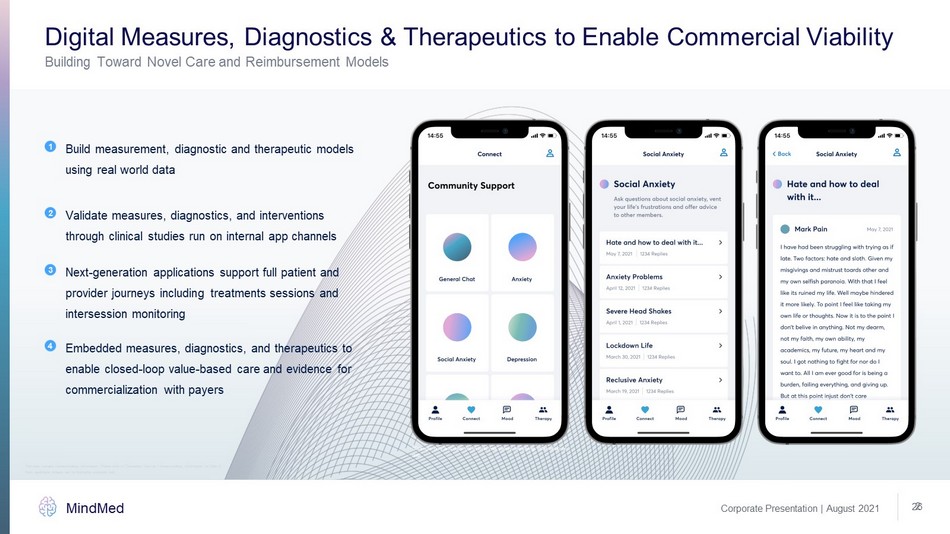
2 Digital Measures, Diagnostics & Therapeutics to Enable Commercial Viability Corporate Presentation | August 2021 MindMed 26 Building Toward Novel Care and Reimbursement Models Build measurement, diagnostic and therapeutic models using real world data 1 Validate measures, diagnostics, and interventions through clinical studies run on internal app channels 2 Next - generation applications support full patient and provider journeys including treatments sessions and intersession monitoring 3 Embedded measures, diagnostics, and therapeutics to enable closed - loop value - based care and evidence for commercialization with payers 4 *Example only and does not include real patient identifying information or data Note: application images are for illustrative purposes only. This slide contains forward - looking information. Please refer to “Cautionary Note on Forward - Looking Information” on Slide 2.
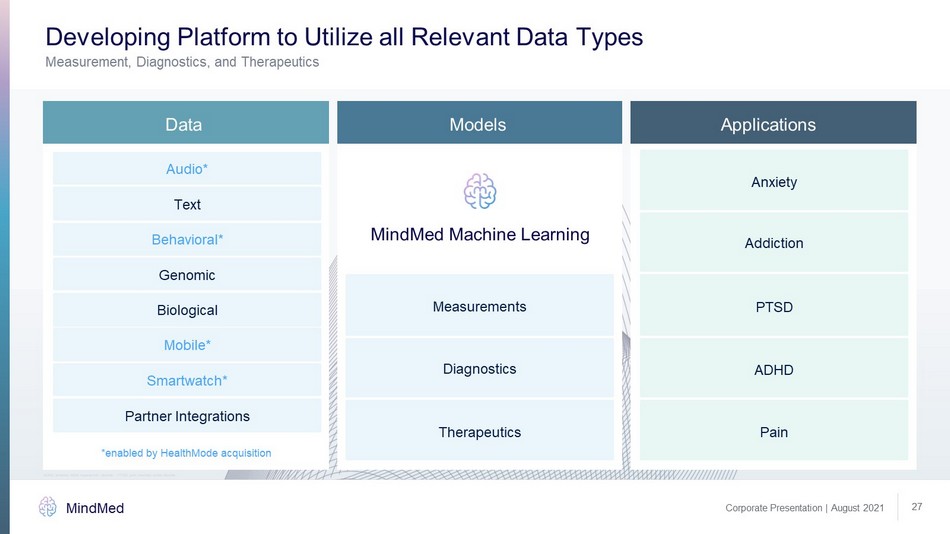
Developing Platform to Utilize all Relevant Data Types MindMed Machine Learning Measurements Audio* Text Behavioral* Genomic Biological Mobile* Smartwatch* Partner Integrations Diagnostics Therapeutics Anxiety Addiction PTSD Pain ADHD Applications Data Models MindMed Corporate Presentation | August 2021 27 *enabled by HealthMode acquisition Measurement, Diagnostics, and Therapeutics ADHD: attention deficit hyperactivity disorder , PTSD: post traumatic stress disorder
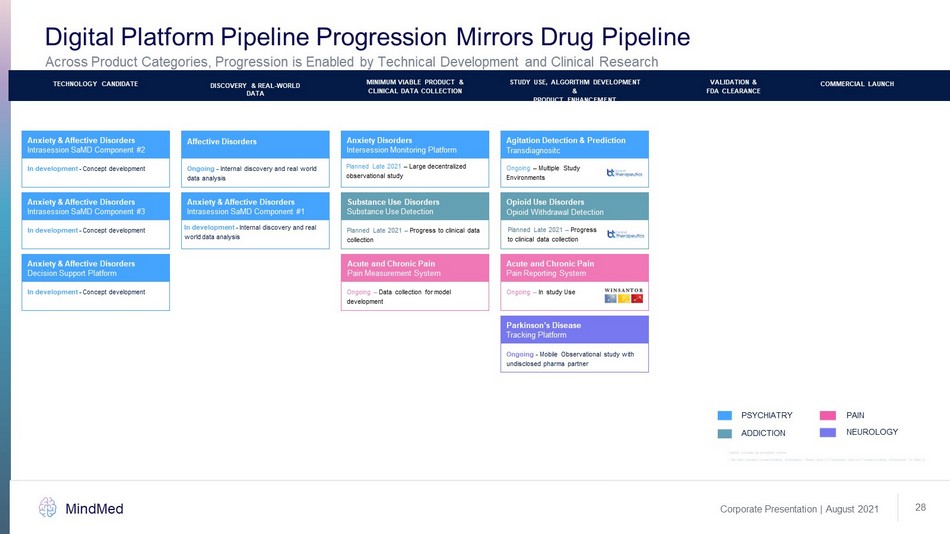
Corporate Presentation | August 2021 MindMed 28 Planned Late 2021 – Progress to clinical data collection Substance Use Disorders Substance Use Detection PAIN NEUROLOGY ADDICTION PSYCHIATRY PRE - CLINICAL PHASE 1 PHASE 2A PHASE 2B PHASE 3 COMMERCIAL LAUNCH In development - Concept development Anxiety & Affective Disorders Decision Support Platform Ongoing - Internal discovery and real world data analysis Affective Disorders In development - Internal discovery and real world data analysis Anxiety & Affective Disorders Intrasession Monitoring Platform In development - Internal discovery real world data analysis Anxiety & Affective Disorders Intrasession SaMD Component #1 In development - Concept development Anxiety & Affective Disorders Intrasession SaMD Component #3 In development - Concept development Anxiety & Affective Disorders Intrasession SaMD Component #2 Planned Late 2021 – Large decentralized observational study Intersession Monitoring Platform Anxiety Disorders DISCOVERY & REAL - WORLD DATA TECHNOLOGY CANDIDATE Ongoing – Multiple Study Environments Transdiagnositc Agitation Detection & Prediction MINIMUM VIABLE PRODUCT & CLINICAL DATA COLLECTION STUDY USE, ALGORITHM DEVELOPMENT & PRODUCT ENHANCEMENT COMMERCIAL LAUNCH VALIDATION & FDA CLEARANCE Ongoing – In study Use Acute and Chronic Pain Pain Reporting System SaMD: software as a medical device Opioid Withdrawal Detection Opioid Use Disorders Planned Late 2021 – Progress to clinical data collection Ongoing – Data collection for model development Acute and Chronic Pain Pain Measurement System Ongoing - Mobile Observational study with undisclosed pharma partner Parkinson’s Disease Tracking Platform Across Product Categories, Progression is Enabled by Technical Development and Clinical Research Digital Platform Pipeline Progression Mirrors Drug Pipeline This slide contains forward - looking information. Please refer to “Cautionary Note on Forward - Looking Information” on Slide 2.

Corporate Presentation | August 2021 MindMed 29 Corporate Information
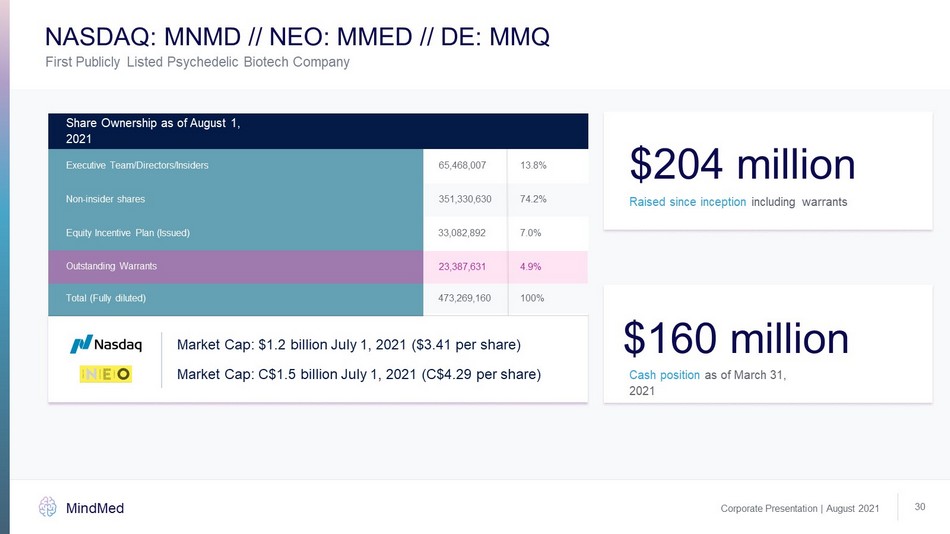
65,468,007 13.8% 74.2% 7.0% 100% Executive Team/Directors/Insiders Share Ownership as of August 1, 2021 Non - insider shares Equity Incentive Plan (Issued) Total (Fully diluted) 351,330,630 33,082,892 473,269,160 $204 million Raised since inception including warrants $160 million Cash position as of March 31, 2021 Corporate Presentation | August 2021 MindMed 30 Market Cap: $1.2 billion July 1, 2021 ($3.41 per share) First Publicly Listed Psychedelic Biotech Company NASDAQ: MNMD // NEO: MMED // DE: MMQ Market Cap: C$1.5 billion July 1, 2021 (C$4.29 per share) 23,387,631 4.9% Outstanding Warrants
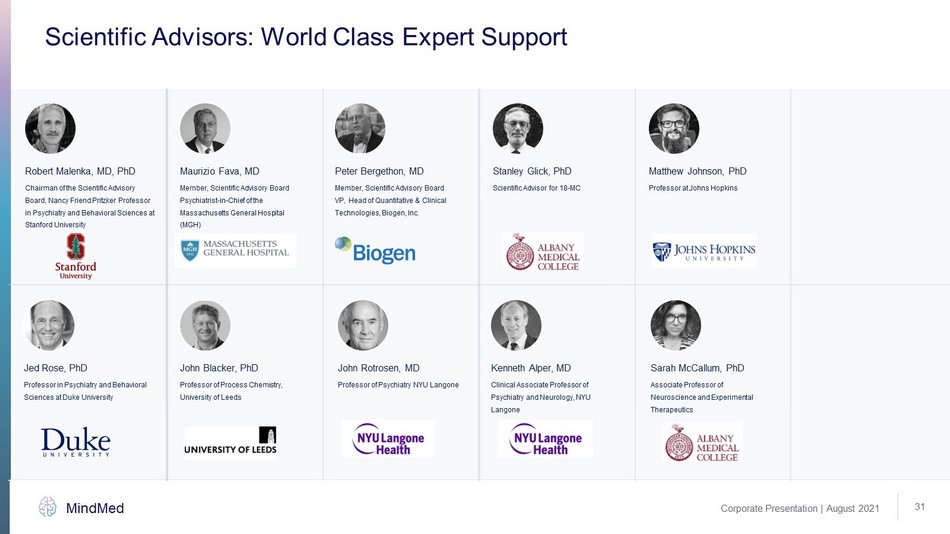
Scientific Advisors: World Class Expert Support Corporate Presentation | August 2021 MindMed 31 Robert Malenka, MD, PhD John Blacker, PhD Jed Rose, PhD Kenneth Alper, MD Maurizio Fava, MD Peter Bergethon, MD Matthew Johnson, PhD Stanley Glick, PhD Sarah McCallum, PhD Chairman of the Scientific Advisory Board, Nancy Friend Pritzker Professor in Psychiatry and Behavioral Sciences at Stanford University Professor of Process Chemistry, University of Leeds Professor in Psychiatry and Behavioral Sciences at Duke University Clinical Associate Professor of Psychiatry and Neurology, NYU Langone Member, Scientific Advisory Board Psychiatrist - in - Chief of the Massachusetts General Hospital (MGH) Member, Scientific Advisory Board VP, Head of Quantitative & Clinical Technologies, Biogen, Inc. Scientific Advisor for 18 - MC Professor at Johns Hopkins Associate Professor of Neuroscience and Experimental Therapeutics John Rotrosen, MD Professor of Psychiatry NYU Langone

Board of Directors Corporate Presentation | August 2021 MindMed 32 Miri Halperin Wernli, PhD Perry Dellelce Bruce Linton Stephen Hurst, JD Brigid Makes Executive President, Director Chairman of the Board of Directors Director, Chair of Compensation, Governance and Nominating Committee Co - Founder and Director Director, Chair of Audit Committee Dr. Halperin Wernli previously worked in clinical psychiatry in Swiss academic hospital settings and then held various global senior leadership positions in the pharma and biotech industries in Switzerland and in the US (Merck, Sharp and Dohme, Roche and Actelion pharmaceuticals) covering Product Development, R&D, and Strategic Marketing. Bruce Linton is an activist Investor with SLANG Worldwide Inc. (CSE:SLNG). Activist Investor with OG DNA Genetics Inc Founder and Former Chairman and CEO of Canopy Growth Corporation (CGC/WEED). Bruce chairs the board’s Compensation, Governance and Nominating Committee. Stephen Hurst has more than thirty - five years’ experience in the biopharmaceutical industry including work for The Immune Tolerance Institute, The Regents of the University of California, The World Bank and BIO Ventures for Global Health. Perry Dellelce is a managing partner of Wildeboer Dellelce LLP. He also serves as chair of the NEO Exchange, Canada’s newest stock exchange. Board Member of Mount Logan Capital Inc. and Lendified Inc. Brigid Makes served as Senior Vice President and Chief Financial Officer of Miramar Labs. Former CFO for Nektar Therapeutics (formerly Inhale Therapeutics) B.A. in Finance and International Business from McGill University and an M.B.A. from Bentley University. Brigid chairs the board’s Audit Committee. Sarah Vinson, MD Director Dr. Vinson is a Triple Board - Certified physician who specializes in adult, child & adolescent, and forensic psychiatry. She is the founder of Lorio Forensics. Dr. Vinson is an Associate Clinical Professor of Psychiatry and Pediatrics at Morehouse School of Medicine, where she is the Program Director of the Child & Adolescent Psychiatry Fellowship, and Adjunct Faculty at Emory University School of Medicine.

Public Perception of Psychedelics Has Changed Corporate Presentation | August 2021 MindMed 33 “Psychedelics - Drug Startup Raises $24 Million Ahead of IPO.” February 27, 2020 “This could save lives, cure depression, help alcoholism, get people off opioids — why wouldn’t I want to be invested?” - Kevin O’ Leary December 9, 2019 “Psychedelic Drug Company MindMed Applies For Nasdaq Up - Listing” September 25, 2020 “Psychedelic drugs may transform mental health care. And big business is ready to profit from the revolution.” February 17, 2020 “The evidence for psychedelics as medicine is far greater than that for CBD, which companies are selling to relieve ills from Parkinson’s to Crohn’s.” April 13, 2020 “New York is getting its first psychedelic - medicine center, with the help of a startup called MindMed, which develops hallucinogens to treat mental illness and addiction, and is funding an institute at N.Y.U. Langone Medical Center.” October 12, 2020 “Its market capitalization of over C$1 billion puts the company ahead of at least eight companies in Canada’s benchmark S&P/TSX Composite Index, according to data compiled by Bloomberg.” December 9, 2020 “A startup that wants to use psychedelics to treat addiction just raised $6.2 million from the host of. Shark Tank and the architect behind the world’s. biggest cannabis grower” September 30, 2019 “MindMed named one of 36 startups that could change the world” December 17, 2019

Corporate Presentation | August 2021 MindMed 34 MindMed
Corporate Presentation | August 2021 MindMed 35 Sources Mental Health By the Numbers | NAMI: National Alliance on Mental Illness. (2019). NAMI. https://www.nami.org/mhstats#:%7E:tex t=2 0.6%25%20of%20U.S.%20adults%20experienced,represents%201%20in%2020%20adult Bandelow, Borwin, and Sophie Michaelis. “Epidemiology of anxiety disorders in the 21st century.” Dialogues in clinical neuros cie nce vol.17,3 (2015): 327 - 35. Weiss, R. D., Potter, J. S., Fiellin, D. A., Byrne, M., Connery, H. S., Dickinson, W., Gardin, J., Griffin, M. L., Gourevitch , M . N., Haller, D. L., Hasson, A. L., Huang, Z., Jacobs, P., Kosinski, A. S., Lindblad, R., McCance - Katz, E. F., Provost, S. E., S elzer, J., Somoza, E. C., Sonne, S. C., … Ling, W. (2011). Adjunctive counseling during brief and extended buprenorphine - naloxone treatment for prescription opioid dependence: a 2 - phase randomized controlled t rial. Archives of general psychiatry, 68(12), 1238 – 1246. https://doi.org/10.1001/archgenpsychiatry.2011.121 Kelly, K. M., & Mezuk, B. (2017). Predictors of remission from generalized anxiety disorder and major depressive disorder. Jo urn al of affective disorders, 208, 467 – 474. https://doi.org/10.1016/j.jad.2016.10.042 Dahlhamer, J., Lucas, J., Zelaya, C., Nahin, R., Mackey, S., DeBar, L., Kerns, R., Von Korff, M., Porter, L., & Helmick, C. ( 201 8). Prevalence of Chronic Pain and High - Impact Chronic Pain Among Adults - United States, 2016. MMWR. Morbidity and mortality we ekly report, 67(36), 1001 – 1006. https://doi.org/10.15585/mmwr.mm6736a2 Hasin DS, Sarvet AL, Meyers JL, et al. Epidemiology of Adult DSM - 5 Major Depressive Disorder and Its Specifiers in the United St ates. JAMA Psychiatry. 2018;75(4):336 – 346. doi:10.1001/jamapsychiatry.2017.4602 2019 National Survey on Drug Use and Health, 2020 Dahlhamer J, Lucas J, Zelaya, C, et al. Prevalence of Chronic Pain and High - Impact Chronic Pain Among Adults — United States, 20 16. MMWR Morb Mortal Wkly Rep 2018;67:1001 – 1006. DOI: http://dx.doi.org/10.15585/mmwr.mm6736a2external icon. Wei DY, Yuan Ong JJ, Goadsby PJ. Cluster Headache: Epidemiology, Pathophysiology, Clinical Features, and Diagnosis. Ann India n A cad Neurol. 2018;21(Suppl 1):S3 - S8. doi:10.4103/aian.AIAN_349_17 Carhart - Harris RL, Leech R, Hellyer PJ, et al. The entropic brain: a theory of conscious states informed by neuroimaging researc h with psychedelic drugs. Front Hum Neurosci. 2014;8:20. Published 2014 Feb 3. doi:10.3389/fnhum.2014.00020 Holze, F., Vizeli, P., Ley, L. et al. Acute dose - dependent effects of lysergic acid diethylamide in a double - blind placebo - contr olled study in healthy subjects. Neuropsychopharmacol. 46, 537 – 544 (2021). https://doi.org/10.1038/s41386 - 020 - 00883 - 6 Carhart - Harris RL, Muthukumaraswamy S, Roseman L, et al. Neural correlates of the LSD experience revealed by multimodal neuroima ging. Proc Natl Acad Sci U S A. 2016;113(17):4853 - 4858. doi:10.1073/pnas.1518377113 Dureja GP, Iyer RN, Das G, Ahdal J, Narang P. Evidence and consensus recommendations for the pharmacological management of pa in in India. J Pain Res. 2017;10:709 - 736. Published 2017 Mar 29. doi:10.2147/JPR.S128655 Ramaekers, J. G., Hutten, N., Mason, N. L., Dolder, P., Theunissen, E. L., Holze, F., Liechti, M. E., Feilding, A., & Kuypers , K . P. (2021). A low dose of lysergic acid diethylamide decreases pain perception in healthy volunteers. Journal of psychopharm aco logy (Oxford, England), 35(4), 398 – 405.13 M.I. (2020, May 6). The U.S. Mental Health Market: $225.1 Billion In Spending In 2019: An OPEN MINDS Market Intelligence Repo rt. OPEN MINDS. https://openminds.com/intelligence - report/the - u - s - mental - health - market - 225 - 1 - billion - in - spending - in - 2019 - an - open - mi nds - market - intelligence - report/