

Corporate Presentation August 2025

This presentation (the “Presentation”) has been prepared by Mind Medicine (MindMed) Inc. (“MindMed”, the “Company”, “we”, “our” or “us) solely for informational purposes. This Presentation does not constitute an offering of, or a solicitation of an offer to purchase, securities of MindMed and under no circumstances is it to be construed as a prospectus or advertisement or public offering of securities. Any trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of the products or services of MindMed. Any amounts are in USD unless otherwise noted. MindMed’s securities have not been approved or disapproved by the Securities and Exchange Commission (the "SEC") or by any state, provincial or other securities regulatory authority, nor has the SEC or any state, provincial or other securities regulatory authority passed on the accuracy or adequacy of this Presentation. Any representation to the contrary is a criminal offense. Cautionary Note Regarding Forward-Looking Statements This Presentation contains, and our officers and representatives may from time to time make, “forward-looking statements” within the meaning of applicable securities laws and are prospective in nature. Forward-looking statements are not based on historical facts, but rather on current expectations and projections about future events and are therefore subject to risks and uncertainties which could cause actual results to differ materially from the future results expressed or implied by the forward-looking statements. These statements generally can be identified by the use of forward-looking words such as “will”, “may", “should”, “could”, “intend”, “estimate”, “plan”, “anticipate”, “expect”, “believe”, “potential”, “continue”, “budget”, “scheduled”, “forecasts”, “intends”, “anticipates”, “projects” or the negative thereof or similar variations. Forward-looking statements in this Presentation include, but are not limited to, statements regarding the anticipated design, timing, progress and results of our investigational programs for MM120, a proprietary, pharmaceutically optimized form of lysergide D-tartrate (including the anticipated topline readouts for the Voyage, Panorama and Emerge studies), MM402, also referred to as R(-)-MDMA, and any other product candidates; the success and timing of our development activities; the success and timing of our planned clinical trials; our ability to meet the milestones set forth herein; the likelihood of success of any clinical trials or of obtaining FDA or other regulatory approvals; our beliefs regarding potential benefits of our product candidates; opinions of potential providers regarding our product candidates, if approved and commercialized; our ability to maximize operational efficiencies through our trial designs; strategies to address drug class methodological considerations; our cash runway funding operations into 2027 based on our current operating plan and anticipated research and development milestones and at least 12 months beyond first Phase 3 topline data readout in GAD; our pre-launch strategy; the potential commercial opportunity for MM120, if approved, including total addressable market; the potential delivery model for MM120, if approved; the potential for the markets that we are anticipating to access and protection of our intellectual property. There are numerous risks and uncertainties that could cause actual results, plans and objectives to differ materially from those expressed in forward-looking statements, including history of negative cash flows, limited operating history, incurrence of future losses, availability of additional capital, compliance with laws and regulations, difficulty associated with research and development, risks associated with clinical trials or studies, heightened regulatory scrutiny, early stage product development, clinical trial risks, regulatory approval processes, novelty of the psychedelic inspired medicines industry, our ability to maintain effective patent rights and other intellectual property protection for our product candidates, our expectations regarding the size of the eligible patient populations for our lead product candidates, if approved and commercialized; our ability to identify third-party treatment sites to conduct our trials and our ability to identify and train appropriate qualified healthcare practitioners to administer our treatments; the pricing, coverage and reimbursement of our lead product candidates, if approved and commercialized; the rate and degree of market acceptance and clinical utility of our lead product candidates, in particular, and controlled substances, in general; as well as those risk factors described in the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2024 under headings such as “Special Note Regarding Forward-Looking Statements,” and “Risk Factors” and “Management's Discussion and Analysis of Financial Condition and Results of Operations” and other filings and furnishings made by the Company with the securities regulatory authorities in all provinces and territories of Canada which are available under the Company's profile on SEDAR+ at www.sedarplus.ca and with the U.S. Securities and Exchange Commission on EDGAR at www.sec.gov. Any forward-looking statement made by MindMed in this Presentation is based only on information currently available to the Company and speaks only as of the date on which it is made. Except as required by law, the Company undertakes no duty or obligation to update any forward-looking statements contained in this Presentation as a result of new information, future events, changes in expectations or otherwise. Cautionary Note Regarding Regulatory Matters The United States federal government regulates drugs through the Controlled Substances Act. MM120 is a proprietary, pharmaceutically optimized form of lysergide D-tartrate and MM402, or R(-)-MDMA, is our proprietary form of the R-enantiomer of MDMA (3,4-methylenedioxymethamphetamine). Lysergide and MDMA are Schedule I substances under the Controlled Substances Act. While the Company is focused on programs using psychedelic or hallucinogenic compounds and non-hallucinogenic derivatives of these compounds, including in its MM120, MM402 and other product candidates, the Company does not have any direct or indirect involvement with the illegal selling, production or distribution of any substances in the jurisdictions in which it operates. The Company is a neuro-pharmaceutical drug development company and does not deal with psychedelic or hallucinogenic substances except within laboratory and clinical trial settings conducted within approved regulatory frameworks. The Company's products will not be commercialized prior to applicable regulatory approval, which will only be granted if clinical evidence of safety and efficacy for the intended uses is successfully developed. Market and Industry Data This Presentation includes market and industry data that has been obtained from third party sources, including industry publications. MindMed believes that the industry data is accurate and that the estimates and assumptions are reasonable, but there is no assurance as to the accuracy or completeness of this data. Third party sources generally state that the information contained therein has been obtained from sources believed to be reliable, but there is no assurance as to the accuracy or completeness of included information. Although the data is believed to be reliable, MindMed has not independently verified any of the data from third party sources referred to in this Presentation or ascertained the underlying economic assumptions relied upon by such sources. References in this Presentation to research reports or to articles and publications should be not construed as depicting the complete findings of the entire referenced report or article. MindMed does not make any representation as to the accuracy of such information. Disclaimer Corporate Presentation | August 2025
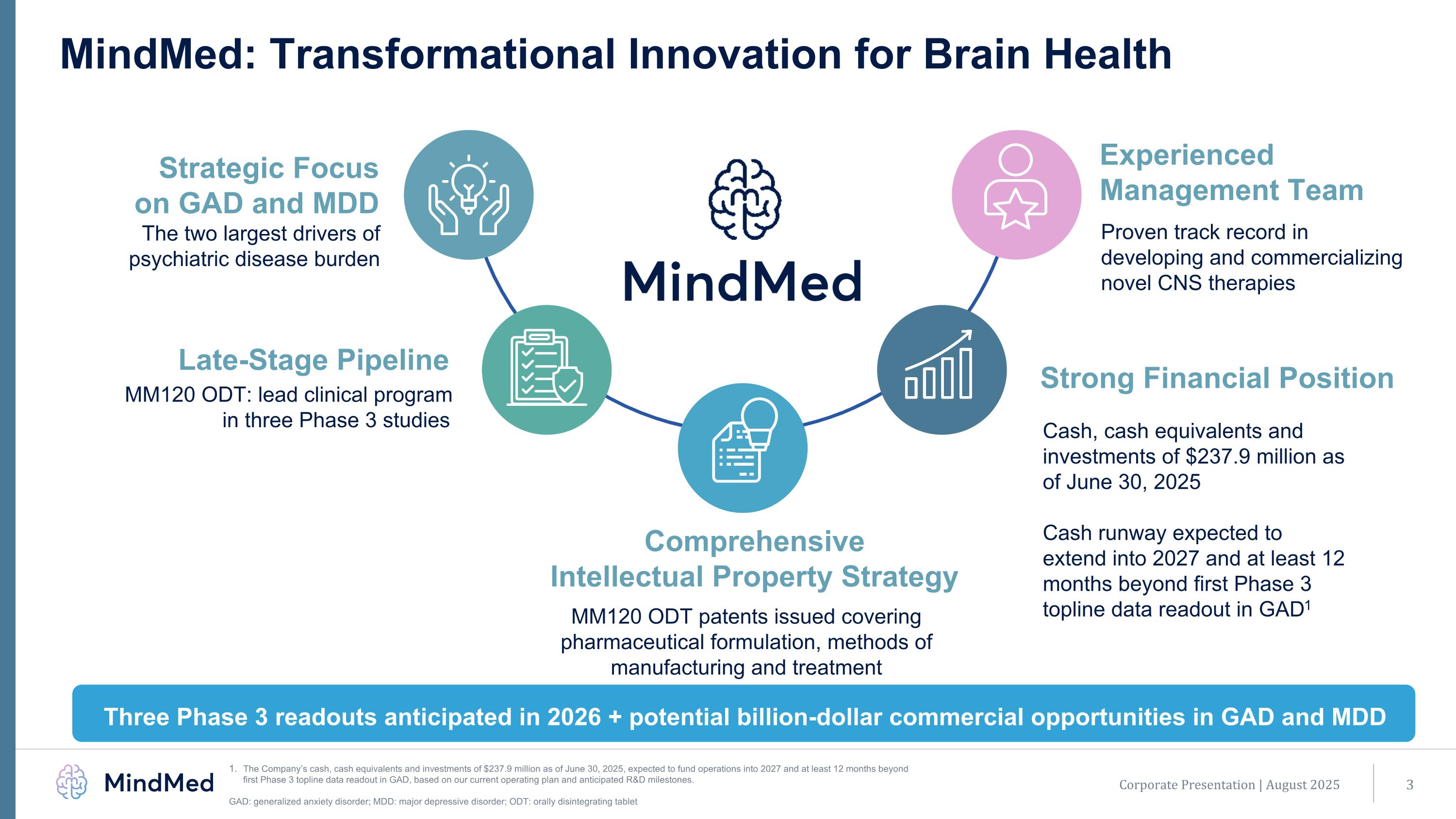
Corporate Presentation | August 2025 MindMed: Transformational Innovation for Brain Health Strategic Focus on GAD and MDD The two largest drivers of psychiatric disease burden Late-Stage Pipeline Comprehensive Intellectual Property Strategy MM120 ODT: lead clinical program in three Phase 3 studies Three Phase 3 readouts anticipated in 2026 + potential billion-dollar commercial opportunities in GAD and MDD MM120 ODT patents issued covering pharmaceutical formulation, methods of manufacturing and treatment Cash, cash equivalents and investments of $237.9 million as of June 30, 2025 Cash runway expected to extend into 2027 and at least 12 months beyond first Phase 3 topline data readout in GAD1 Strong Financial Position Experienced Management Team Proven track record in developing and commercializing novel CNS therapies The Company’s cash, cash equivalents and investments of $237.9 million as of June 30, 2025, expected to fund operations into 2027 and at least 12 months beyond first Phase 3 topline data readout in GAD, based on our current operating plan and anticipated R&D milestones. GAD: generalized anxiety disorder; MDD: major depressive disorder; ODT: orally disintegrating tablet

2025 On Track and Executing MM120-300 for GAD Phase 3 topline readout 1H 2026 ANTICIPATED MILESTONES MM120-301 for GAD Phase 3 topline readout 2H 2026 MM120-310 for MDD Phase 3 topline readout 2H 2026
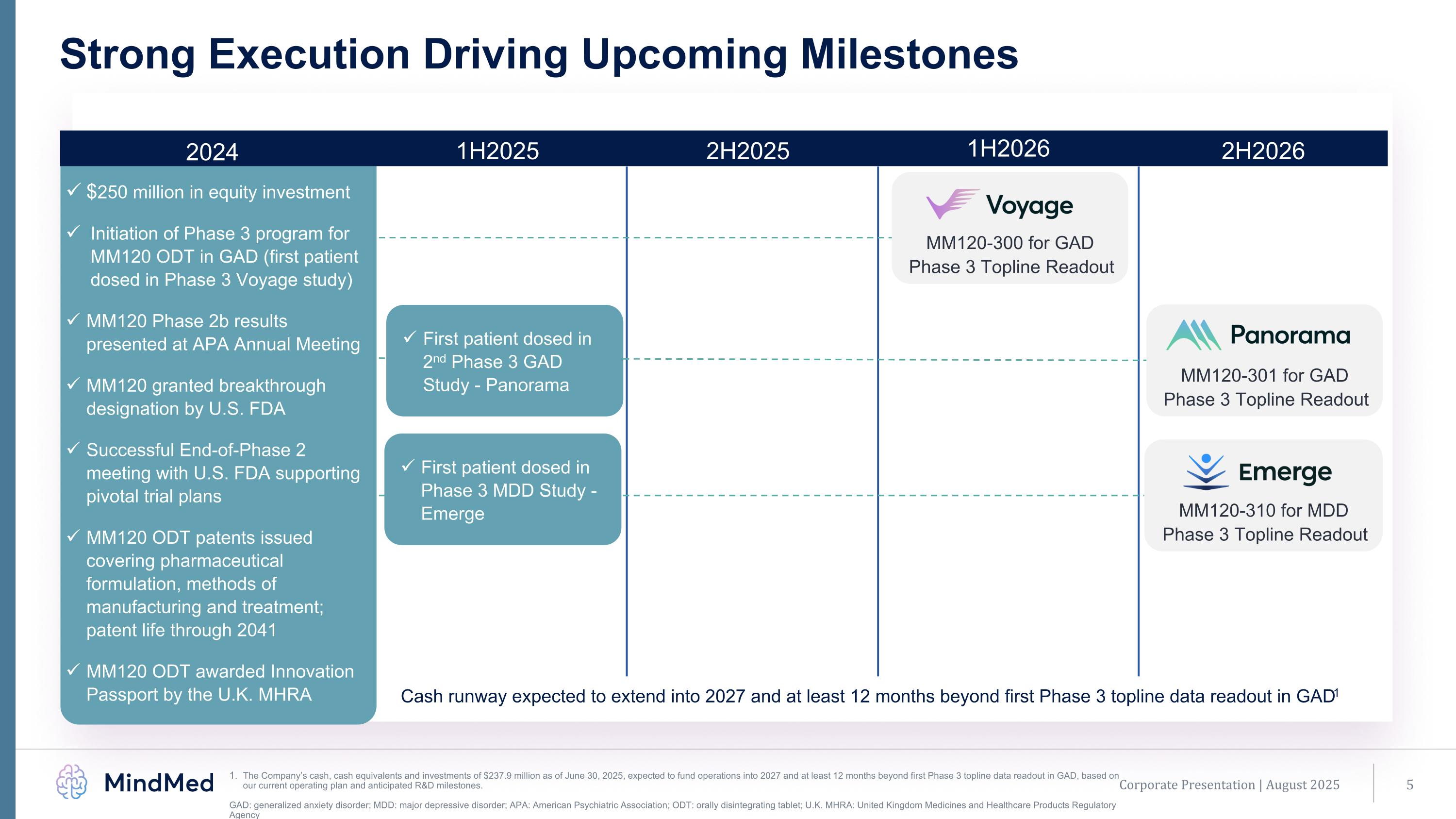
2024 1H2025 2H2025 1H2026 2H2026 MM120-310 for MDD Phase 3 Topline Readout MM120-301 for GAD Phase 3 Topline Readout Cash runway expected to extend into 2027 and at least 12 months beyond first Phase 3 topline data readout in GAD1 $250 million in equity investment Initiation of Phase 3 program for MM120 ODT in GAD (first patient dosed in Phase 3 Voyage study) MM120 Phase 2b results presented at APA Annual Meeting MM120 granted breakthrough designation by U.S. FDA Successful End-of-Phase 2 meeting with U.S. FDA supporting pivotal trial plans MM120 ODT patents issued covering pharmaceutical formulation, methods of manufacturing and treatment; patent life through 2041 MM120 ODT awarded Innovation Passport by the U.K. MHRA Corporate Presentation | August 2025 Strong Execution Driving Upcoming Milestones The Company’s cash, cash equivalents and investments of $237.9 million as of June 30, 2025, expected to fund operations into 2027 and at least 12 months beyond first Phase 3 topline data readout in GAD, based on our current operating plan and anticipated R&D milestones. GAD: generalized anxiety disorder; MDD: major depressive disorder; APA: American Psychiatric Association; ODT: orally disintegrating tablet; U.K. MHRA: United Kingdom Medicines and Healthcare Products Regulatory Agency First patient dosed in 2nd Phase 3 GAD Study - Panorama First patient dosed in Phase 3 MDD Study - Emerge MM120-300 for GAD Phase 3 Topline Readout
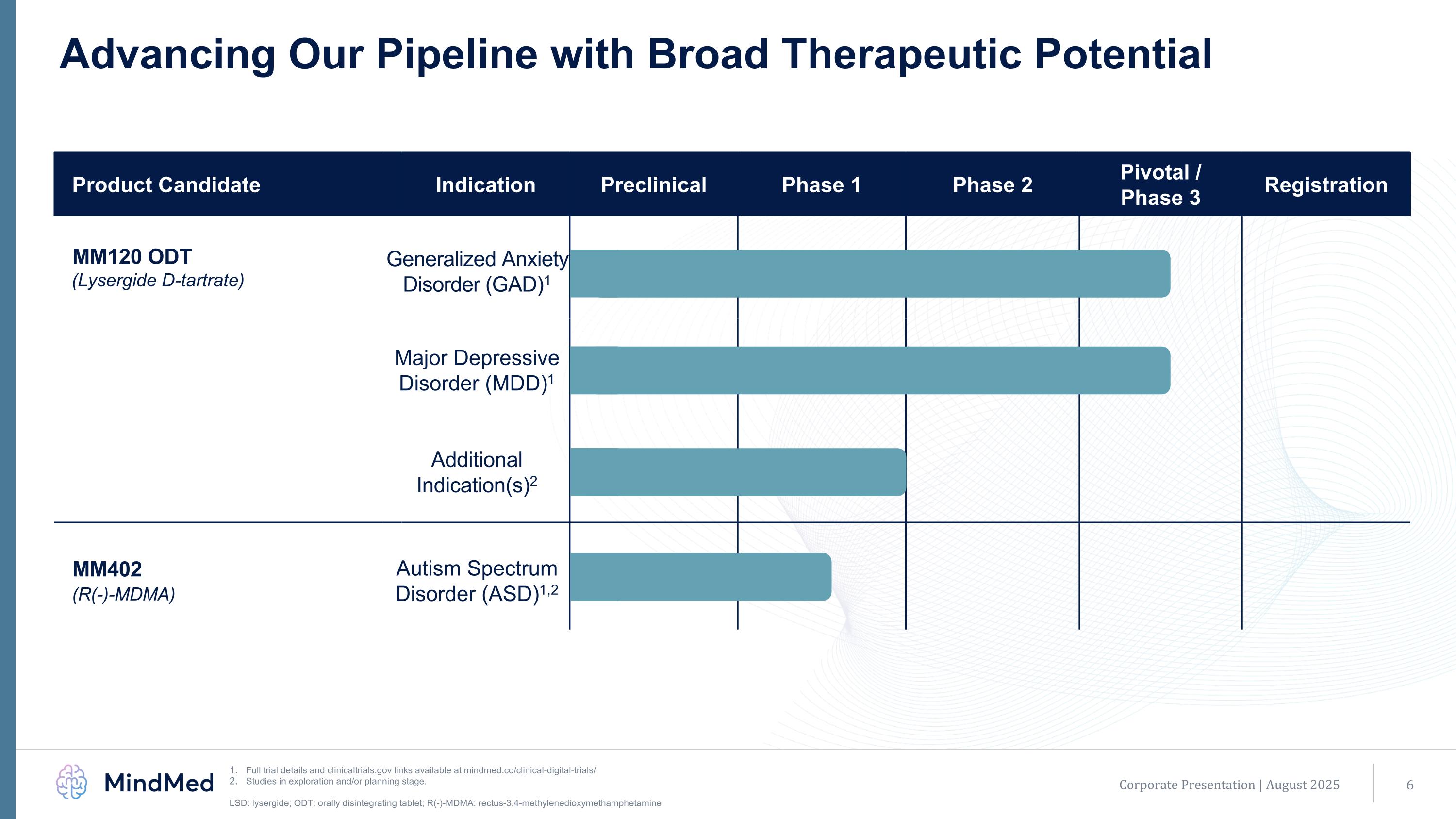
Advancing Our Pipeline with Broad Therapeutic Potential Full trial details and clinicaltrials.gov links available at mindmed.co/clinical-digital-trials/ Studies in exploration and/or planning stage. LSD: lysergide; ODT: orally disintegrating tablet; R(-)-MDMA: rectus-3,4-methylenedioxymethamphetamine Corporate Presentation | August 2025 Product Candidate Indication Preclinical Phase 1 Phase 2 Pivotal / Phase 3 Registration MM120 ODT (Lysergide D-tartrate) Generalized Anxiety Disorder (GAD)1 Generalized Anxiety Disorder (GAD) Major Depressive Disorder (MDD)1 Additional Indication(s)2 Additional Psychiatric Indication MM402 (R(-)-MDMA) Autism Spectrum Disorder (ASD)1,2 Autism Spectrum Disorder (ASD)
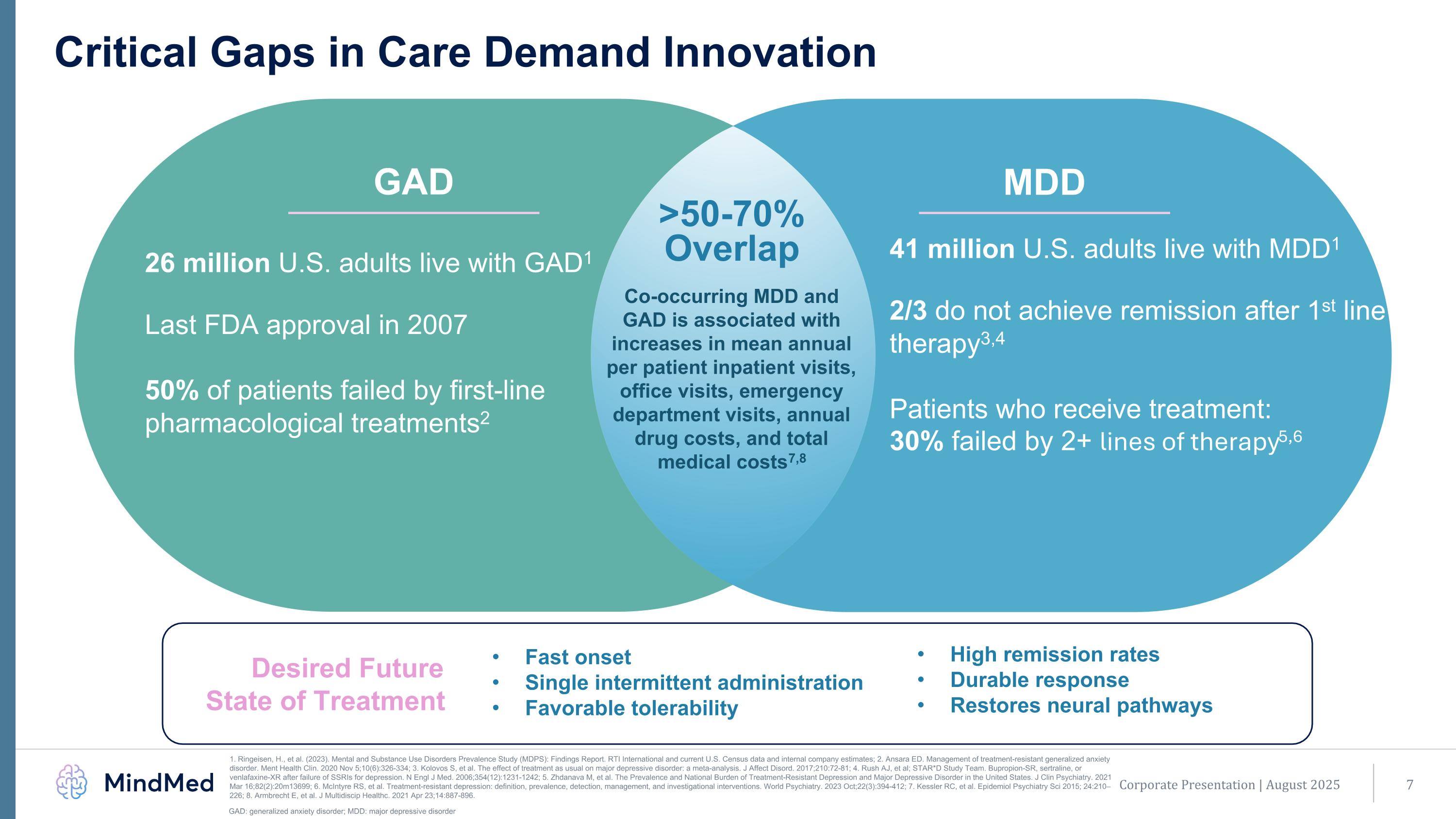
Corporate Presentation | August 2025 Critical Gaps in Care Demand Innovation Fast onset Single intermittent administration Favorable tolerability 26 million U.S. adults live with GAD1 Last FDA approval in 2007 50% of patients failed by first-line pharmacological treatments2 Co-occurring MDD and GAD is associated with increases in mean annual per patient inpatient visits, office visits, emergency department visits, annual drug costs, and total medical costs7,8 41 million U.S. adults live with MDD1 2/3 do not achieve remission after 1st line therapy3,4 Patients who receive treatment: 30% failed by 2+ lines of therapy5,6 MDD >50-70% Overlap GAD High remission rates Durable response Restores neural pathways Desired Future State of Treatment GAD: generalized anxiety disorder; MDD: major depressive disorder 1. Ringeisen, H., et al. (2023). Mental and Substance Use Disorders Prevalence Study (MDPS): Findings Report. RTI International and current U.S. Census data and internal company estimates; 2. Ansara ED. Management of treatment-resistant generalized anxiety disorder. Ment Health Clin. 2020 Nov 5;10(6):326-334; 3. Kolovos S, et al. The effect of treatment as usual on major depressive disorder: a meta-analysis. J Affect Disord. 2017;210:72-81; 4. Rush AJ, et al; STAR*D Study Team. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231-1242; 5. Zhdanava M, et al. The Prevalence and National Burden of Treatment-Resistant Depression and Major Depressive Disorder in the United States. J Clin Psychiatry. 2021 Mar 16;82(2):20m13699; 6. McIntyre RS, et al. Treatment-resistant depression: definition, prevalence, detection, management, and investigational interventions. World Psychiatry. 2023 Oct;22(3):394-412; 7. Kessler RC, et al. Epidemiol Psychiatry Sci 2015; 24:210–226; 8. Armbrecht E, et al. J Multidiscip Healthc. 2021 Apr 23;14:887-896.
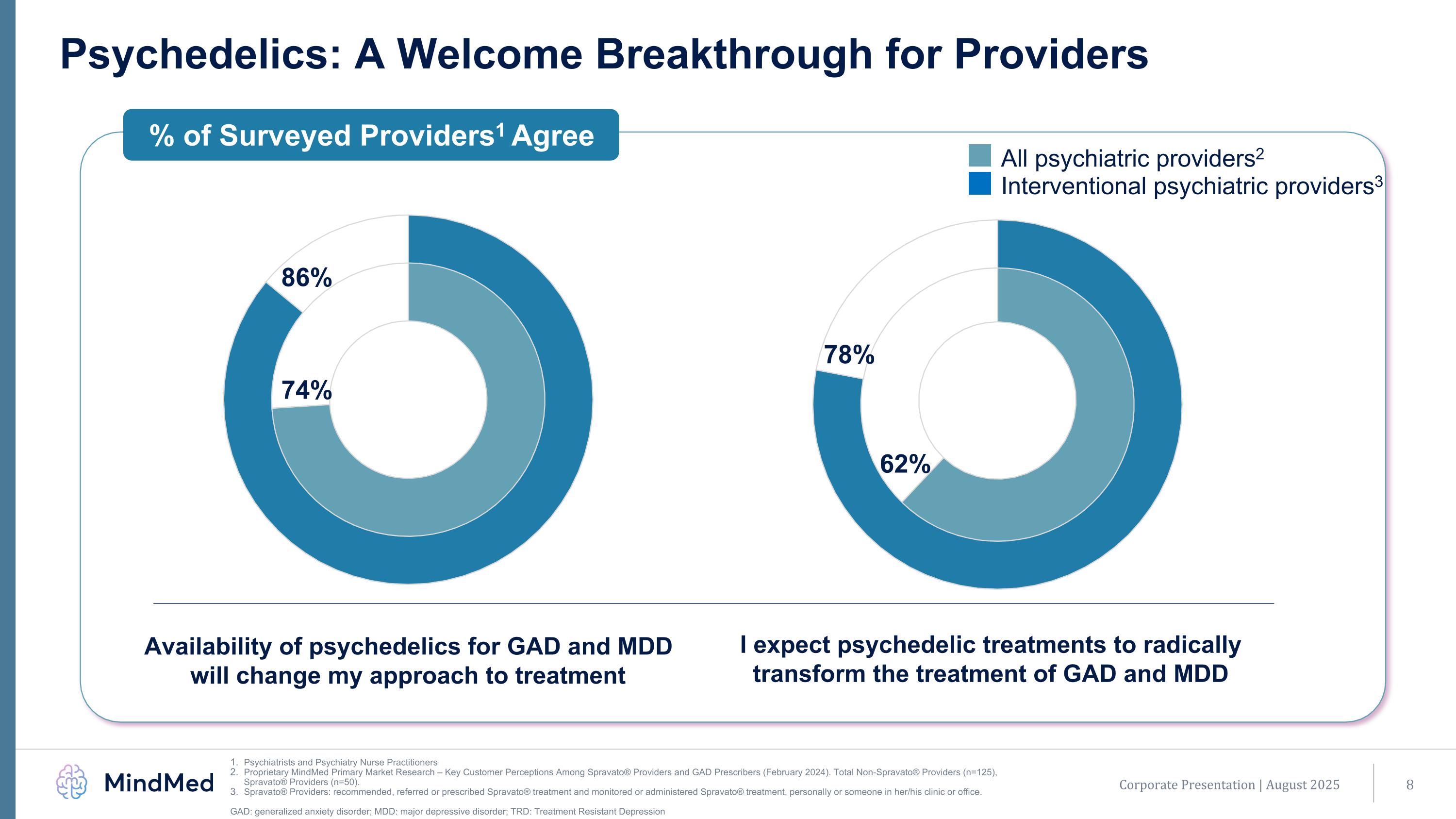
Psychedelics: A Welcome Breakthrough for Providers Psychiatrists and Psychiatry Nurse Practitioners Proprietary MindMed Primary Market Research – Key Customer Perceptions Among Spravato® Providers and GAD Prescribers (February 2024). Total Non-Spravato® Providers (n=125), Spravato® Providers (n=50). Spravato® Providers: recommended, referred or prescribed Spravato® treatment and monitored or administered Spravato® treatment, personally or someone in her/his clinic or office. GAD: generalized anxiety disorder; MDD: major depressive disorder; TRD: Treatment Resistant Depression % of Surveyed Providers1 Agree All psychiatric providers2 Interventional psychiatric providers3 Availability of psychedelics for GAD and MDD will change my approach to treatment I expect psychedelic treatments to radically transform the treatment of GAD and MDD 62% 78% 74% 86% Corporate Presentation | August 2025

Program Overview MM120 ODT Lysergide D-tartrate
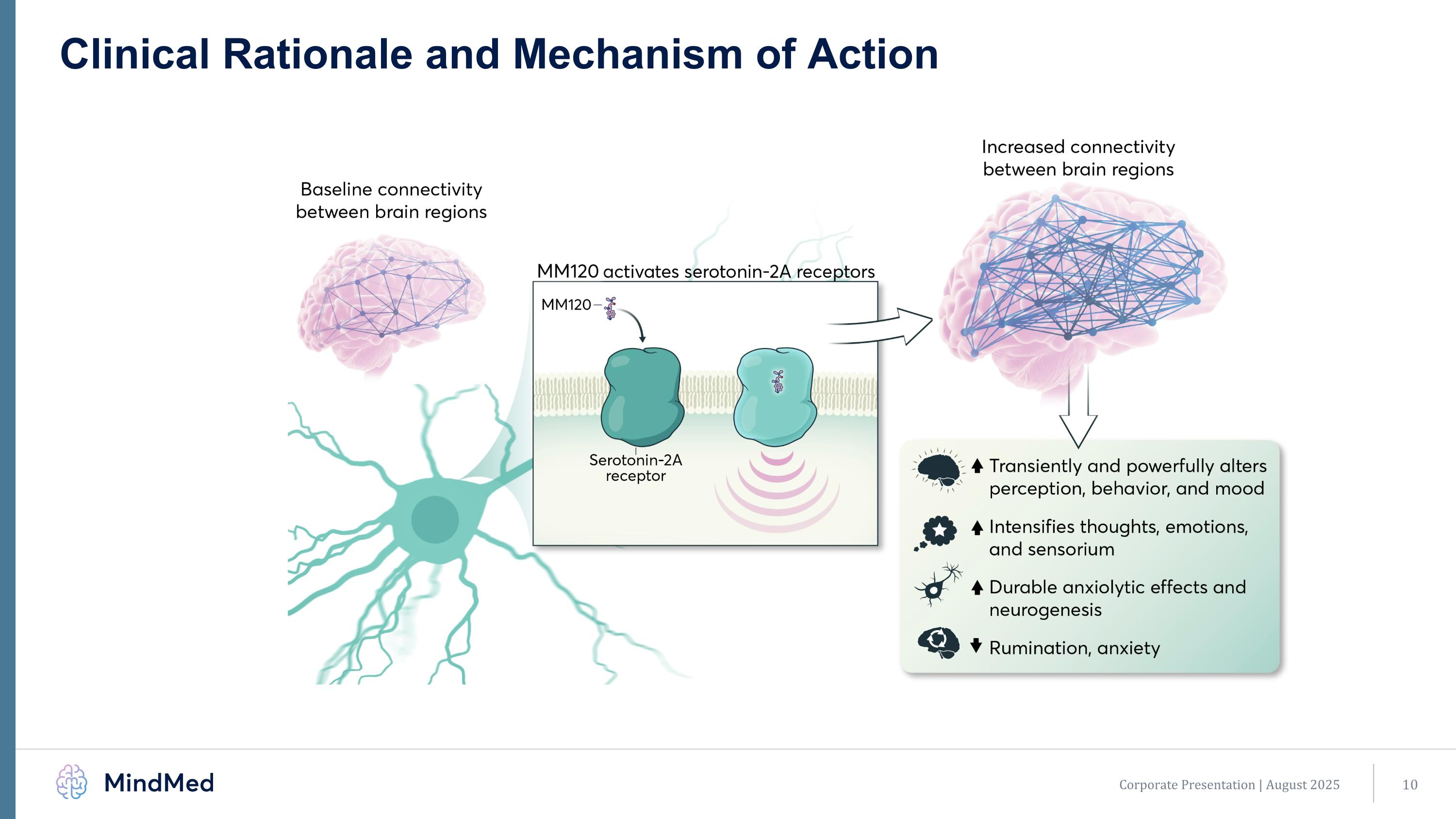
Clinical Rationale and Mechanism of Action Corporate Presentation | August 2025
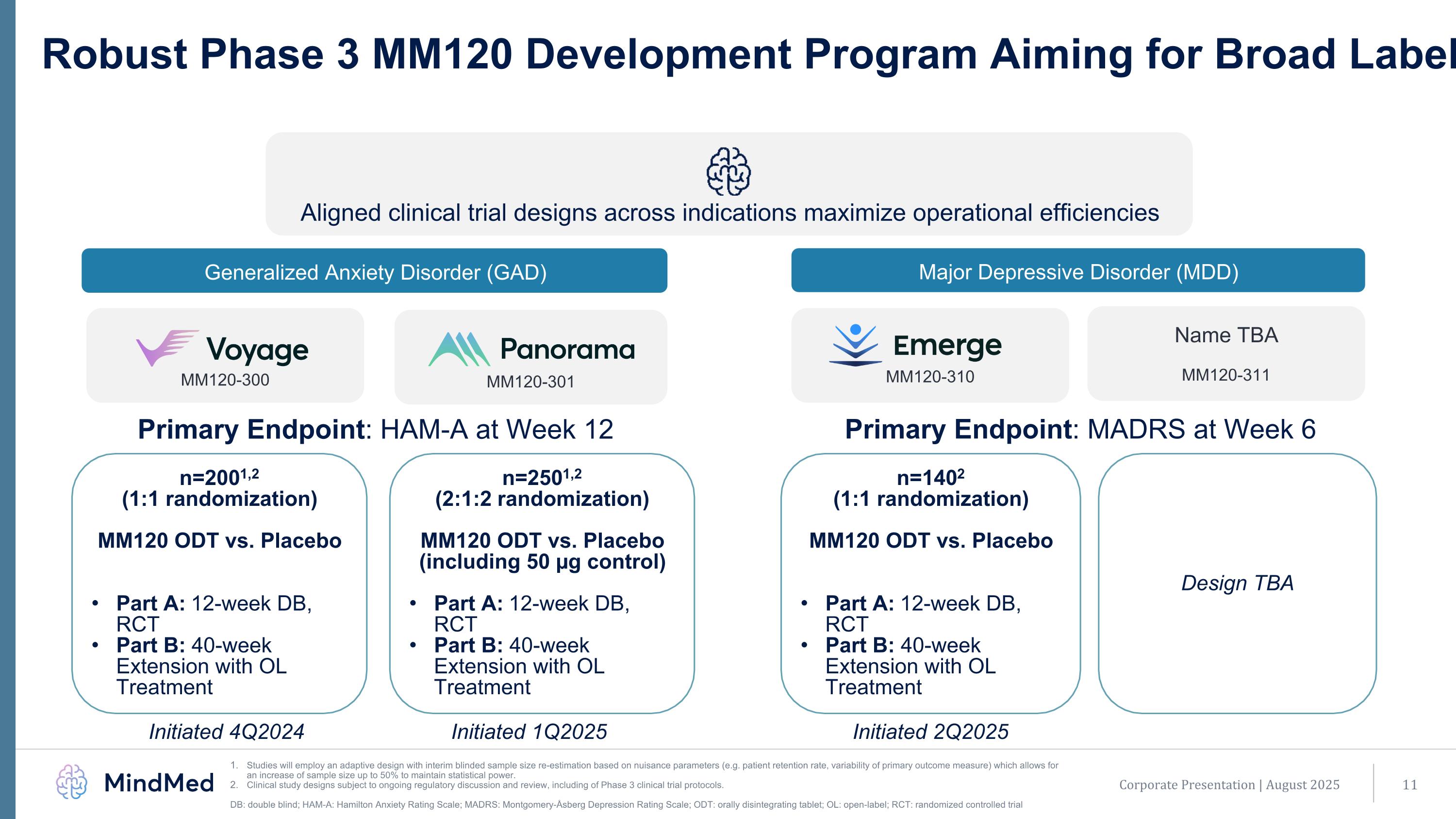
Robust Phase 3 MM120 Development Program Aiming for Broad Label Studies will employ an adaptive design with interim blinded sample size re-estimation based on nuisance parameters (e.g. patient retention rate, variability of primary outcome measure) which allows for an increase of sample size up to 50% to maintain statistical power. Clinical study designs subject to ongoing regulatory discussion and review, including of Phase 3 clinical trial protocols. DB: double blind; HAM-A: Hamilton Anxiety Rating Scale; MADRS: Montgomery-Åsberg Depression Rating Scale; ODT: orally disintegrating tablet; OL: open-label; RCT: randomized controlled trial MM120-300 MM120-301 Aligned clinical trial designs across indications maximize operational efficiencies Generalized Anxiety Disorder (GAD) Major Depressive Disorder (MDD) MM120-310 n=2001,2 (1:1 randomization) MM120 ODT vs. Placebo Part A: 12-week DB, RCT Part B: 40-week Extension with OL Treatment n=2501,2 (2:1:2 randomization) MM120 ODT vs. Placebo (including 50 µg control) Part A: 12-week DB, RCT Part B: 40-week Extension with OL Treatment n=1402 (1:1 randomization) MM120 ODT vs. Placebo Part A: 12-week DB, RCT Part B: 40-week Extension with OL Treatment Initiated 4Q2024 Initiated 1Q2025 Initiated 2Q2025 Name TBA MM120-311 Design TBA Primary Endpoint: HAM-A at Week 12 Primary Endpoint: MADRS at Week 6 Corporate Presentation | August 2025
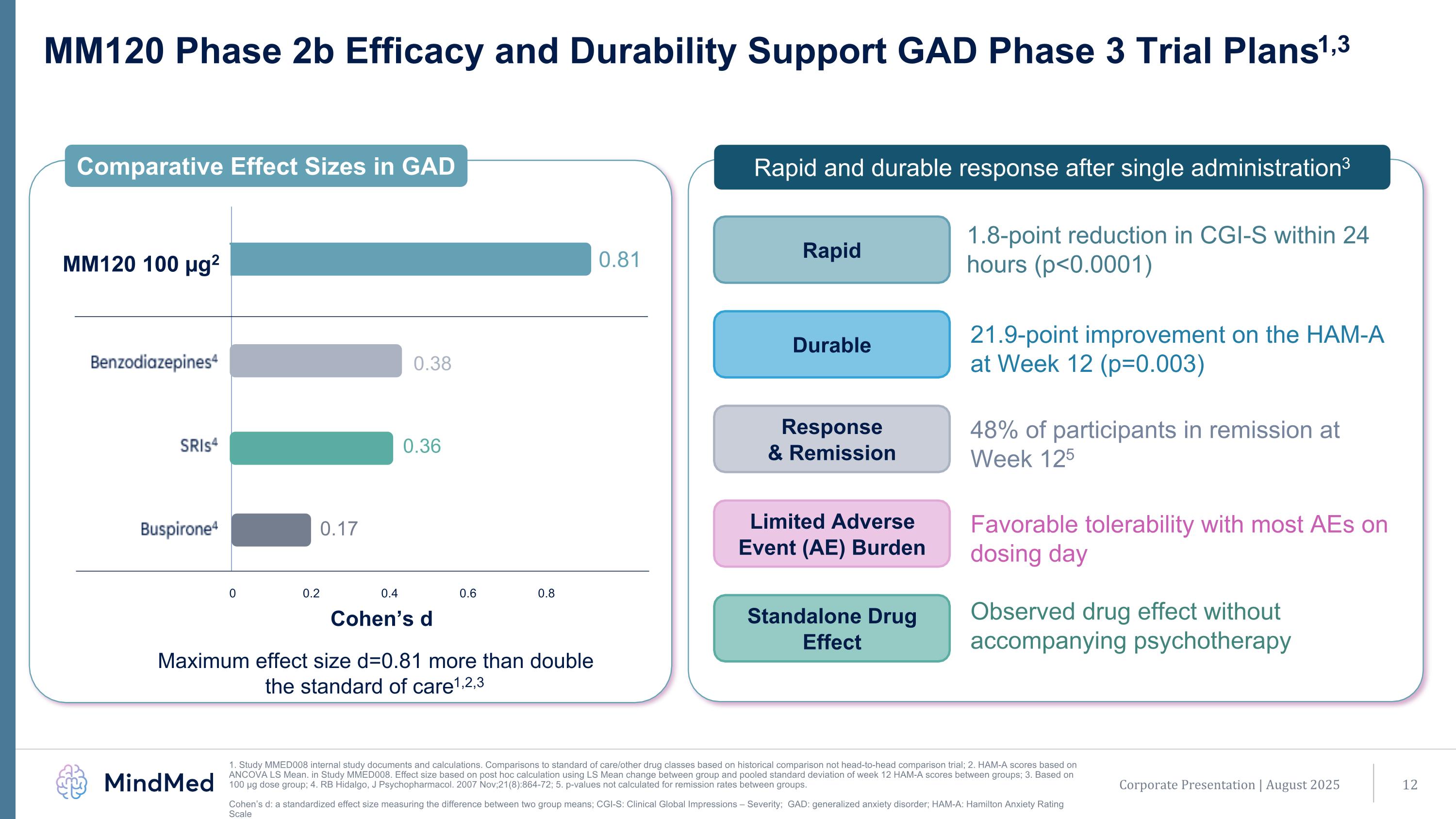
MM120 Phase 2b Efficacy and Durability Support GAD Phase 3 Trial Plans1,3 1. Study MMED008 internal study documents and calculations. Comparisons to standard of care/other drug classes based on historical comparison not head-to-head comparison trial; 2. HAM-A scores based on ANCOVA LS Mean. in Study MMED008. Effect size based on post hoc calculation using LS Mean change between group and pooled standard deviation of week 12 HAM-A scores between groups; 3. Based on 100 µg dose group; 4. RB Hidalgo, J Psychopharmacol. 2007 Nov;21(8):864-72; 5. p-values not calculated for remission rates between groups. Cohen’s d: a standardized effect size measuring the difference between two group means; CGI-S: Clinical Global Impressions – Severity; GAD: generalized anxiety disorder; HAM-A: Hamilton Anxiety Rating Scale Maximum effect size d=0.81 more than double the standard of care1,2,3 0 0.2 0.4 0.6 0.8 0.38 0.36 0.17 0.81 Comparative Effect Sizes in GAD Rapid and durable response after single administration3 Rapid Response & Remission Durable Standalone Drug Effect Limited Adverse Event (AE) Burden 1.8-point reduction in CGI-S within 24 hours (p<0.0001) 21.9-point improvement on the HAM-A at Week 12 (p=0.003) 48% of participants in remission at Week 125 Favorable tolerability with most AEs on dosing day Observed drug effect without accompanying psychotherapy Cohen’s d Corporate Presentation | August 2025 MM120 100 µg2 Benzodiazepines4 SRIs4 Buspirone4
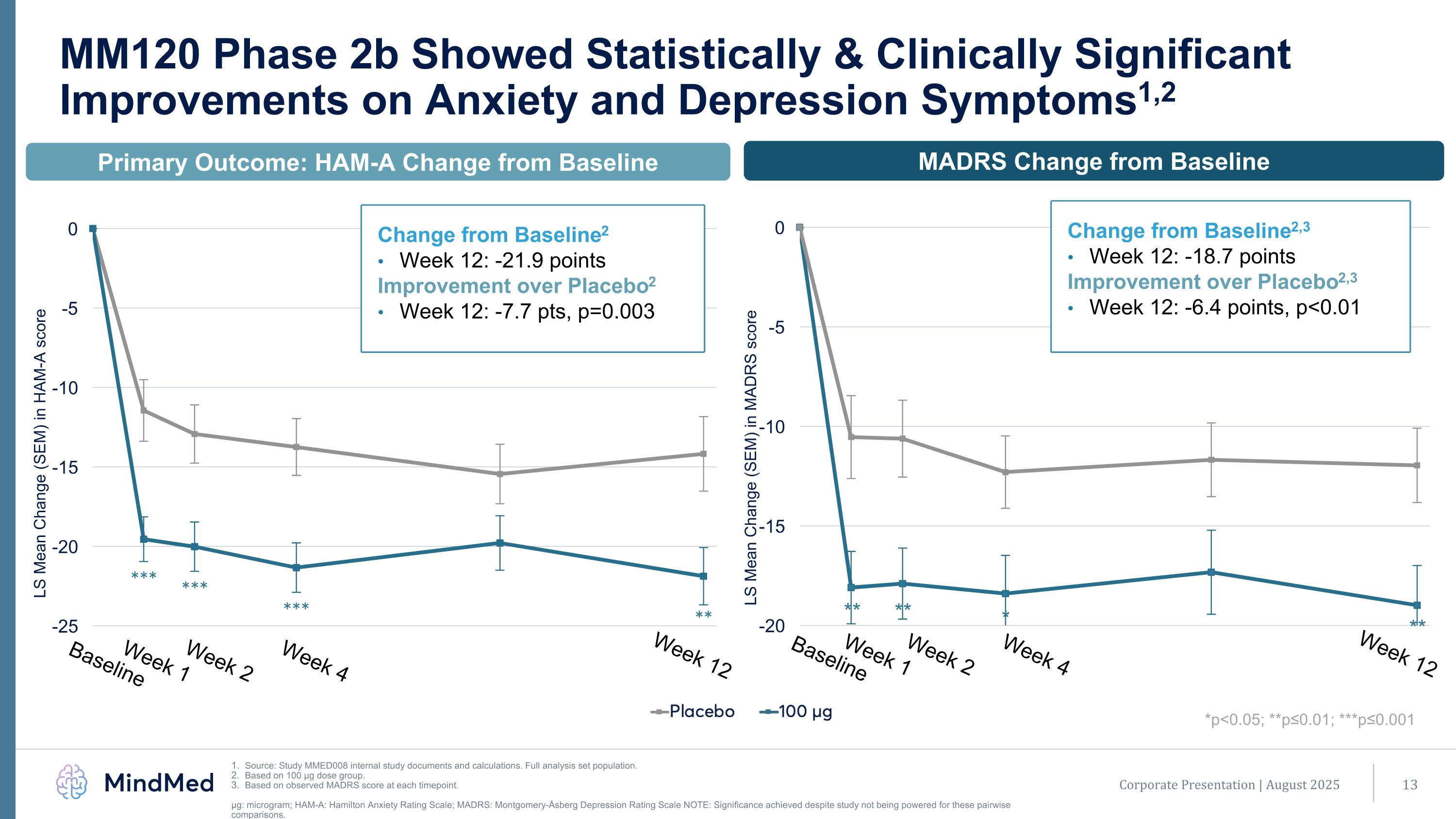
Primary Outcome: HAM-A Change from Baseline MM120 Phase 2b Showed Statistically & Clinically Significant Improvements on Anxiety and Depression Symptoms1,2 Source: Study MMED008 internal study documents and calculations. Full analysis set population. Based on 100 µg dose group. Based on observed MADRS score at each timepoint. μg: microgram; HAM-A: Hamilton Anxiety Rating Scale; MADRS: Montgomery-Åsberg Depression Rating Scale NOTE: Significance achieved despite study not being powered for these pairwise comparisons. *p<0.05; **p≤0.01; ***p≤0.001 Baseline Week 1 Week 2 Week 4 Week 12 *** *** *** ** MADRS Change from Baseline ** ** * ** Change from Baseline2 Week 12: -21.9 points Improvement over Placebo2 Week 12: -7.7 pts, p=0.003 Change from Baseline2,3 Week 12: -18.7 points Improvement over Placebo2,3 Week 12: -6.4 points, p<0.01 Baseline Week 1 Week 2 Week 4 Week 12 Corporate Presentation | August 2025
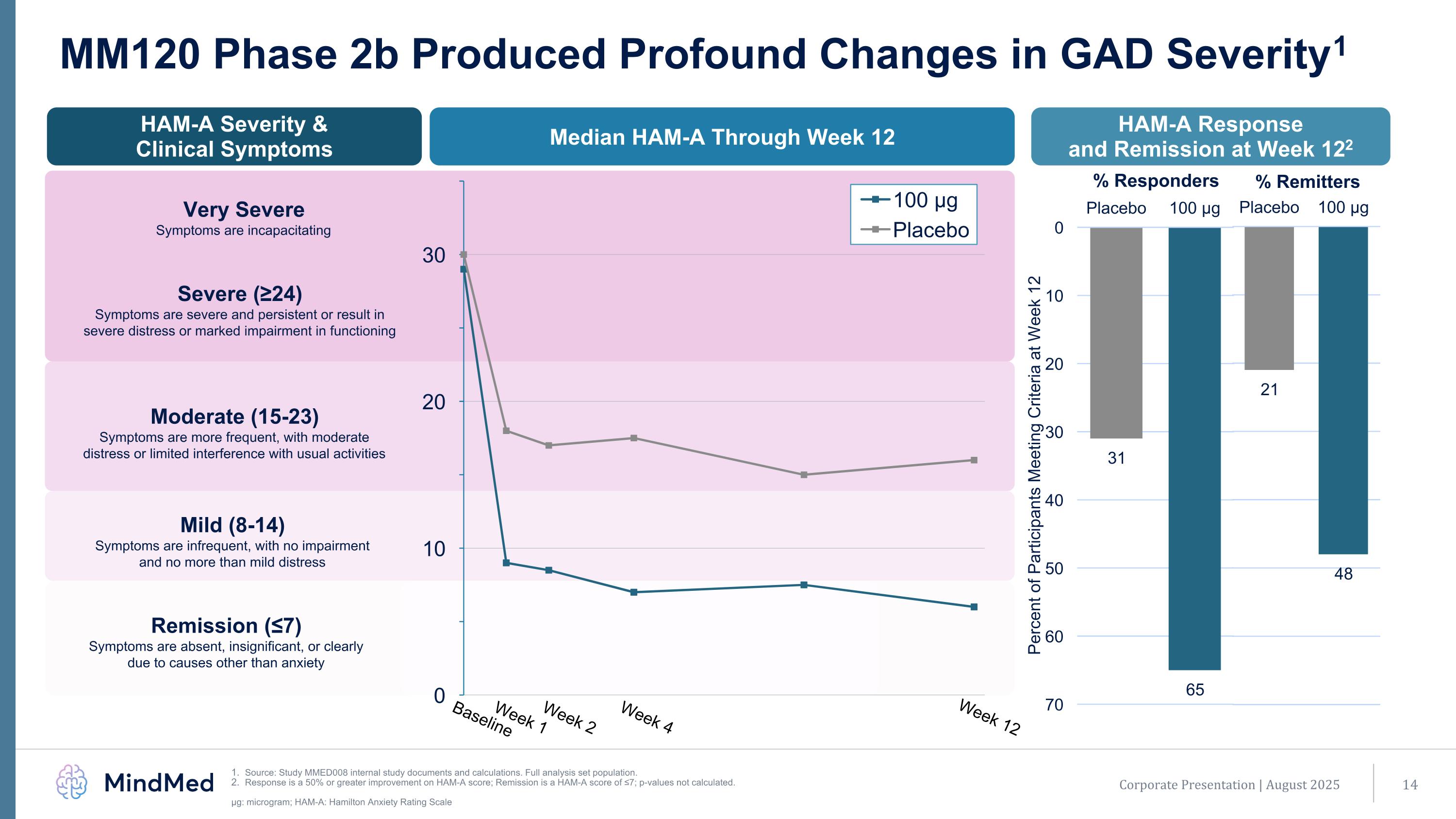
MM120 Phase 2b Produced Profound Changes in GAD Severity1 Source: Study MMED008 internal study documents and calculations. Full analysis set population. Response is a 50% or greater improvement on HAM-A score; Remission is a HAM-A score of ≤7; p-values not calculated. μg: microgram; HAM-A: Hamilton Anxiety Rating Scale HAM-A Response and Remission at Week 122 Median HAM-A Through Week 12 HAM-A Severity & Clinical Symptoms Very Severe Symptoms are incapacitating Moderate (15-23) Symptoms are more frequent, with moderate distress or limited interference with usual activities Mild (8-14) Symptoms are infrequent, with no impairment and no more than mild distress Remission (≤7) Symptoms are absent, insignificant, or clearly due to causes other than anxiety Baseline Week 1 Week 2 Week 4 Week 12 Corporate Presentation | August 2025 Severe (≥24) Symptoms are severe and persistent or result in severe distress or marked impairment in functioning
MM120 Phase 2b was Well-tolerated with Mostly Expected Transient, Mild-to-Moderate Adverse Events on Dosing Day1 Source: Study MMED008 internal study documents and calculations. Safety population. One serious adverse event (SAE) was observed in the 50 µg dose group: panic attack on study day 98 that was deemed not related to treatment. Suicidality assessment based on reported adverse events. Virtually all (99%) adverse events (AEs) were mild-to-moderate in severity Minimal (2.5%) treatment emergent AEs (TEAEs) led to study withdrawal No drug-related serious AEs (SAEs)2 Favorable tolerability profile No SAEs related to study drug No suicidal behavior or suicidality signal3 Only SAE was in 50 µg dose group and deemed unrelated 2 AE profile consistent with historical studies and drug class No suicidal or self-injurious behavior No indication of increased suicidality or suicide-related risk ≤2 participants per arm reported suicidal ideation during the study Corporate Presentation | August 2025
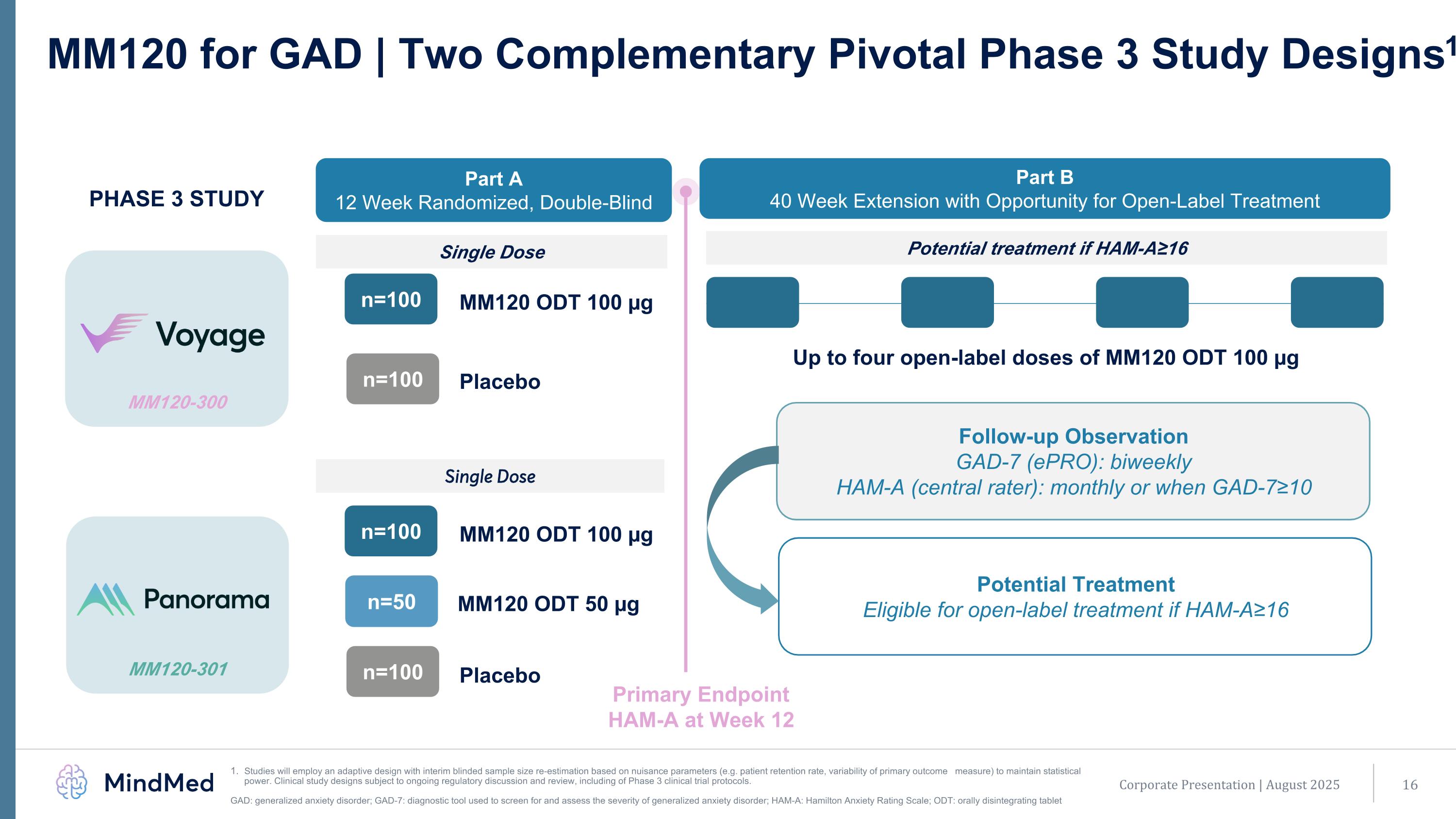
MM120-301 MM120-300 MM120 for GAD | Two Complementary Pivotal Phase 3 Study Designs1 Studies will employ an adaptive design with interim blinded sample size re-estimation based on nuisance parameters (e.g. patient retention rate, variability of primary outcome measure) to maintain statistical power. Clinical study designs subject to ongoing regulatory discussion and review, including of Phase 3 clinical trial protocols. GAD: generalized anxiety disorder; GAD-7: diagnostic tool used to screen for and assess the severity of generalized anxiety disorder; HAM-A: Hamilton Anxiety Rating Scale; ODT: orally disintegrating tablet MM120 ODT 100 µg n=100 Placebo n=100 Part A 12 Week Randomized, Double-Blind Part B 40 Week Extension with Opportunity for Open-Label Treatment MM120 ODT 100 µg n=100 Placebo n=100 MM120 ODT 50 µg n=50 PHASE 3 STUDY Single Dose Potential treatment if HAM-A≥16 Primary Endpoint HAM-A at Week 12 Up to four open-label doses of MM120 ODT 100 µg Single Dose Follow-up Observation GAD-7 (ePRO): biweekly HAM-A (central rater): monthly or when GAD-7≥10 Potential Treatment Eligible for open-label treatment if HAM-A≥16 Corporate Presentation | August 2025
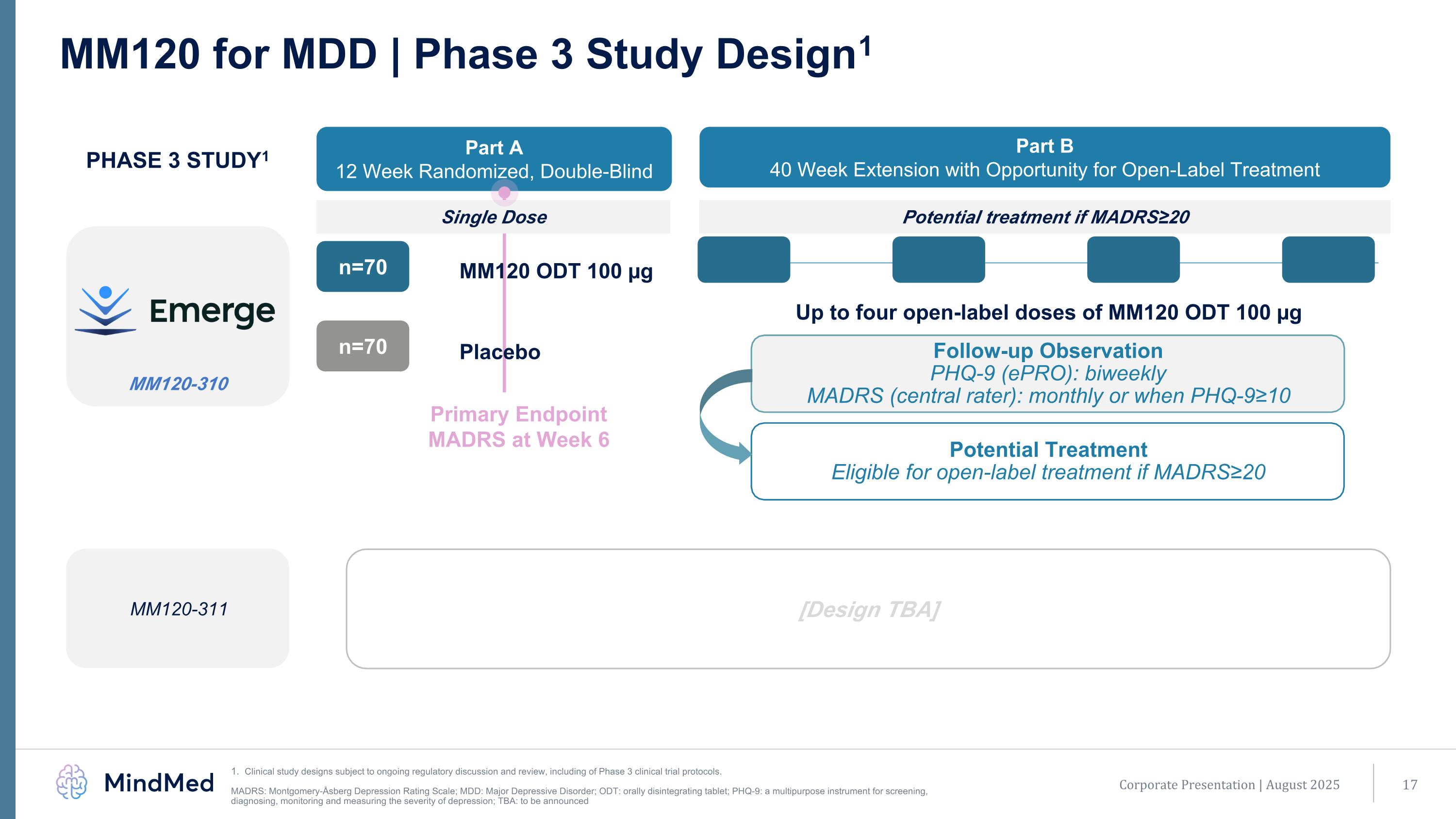
Primary Endpoint MADRS at Week 6 MM120-311 MM120-310 MM120 for MDD | Phase 3 Study Design1 Clinical study designs subject to ongoing regulatory discussion and review, including of Phase 3 clinical trial protocols. MADRS: Montgomery-Åsberg Depression Rating Scale; MDD: Major Depressive Disorder; ODT: orally disintegrating tablet; PHQ-9: a multipurpose instrument for screening, diagnosing, monitoring and measuring the severity of depression; TBA: to be announced MM120 ODT 100 µg n=70 Placebo n=70 Part A 12 Week Randomized, Double-Blind Part B 40 Week Extension with Opportunity for Open-Label Treatment PHASE 3 STUDY1 Single Dose Potential treatment if MADRS≥20 Up to four open-label doses of MM120 ODT 100 µg [Design TBA] Follow-up Observation PHQ-9 (ePRO): biweekly MADRS (central rater): monthly or when PHQ-9≥10 Potential Treatment Eligible for open-label treatment if MADRS≥20 Corporate Presentation | August 2025
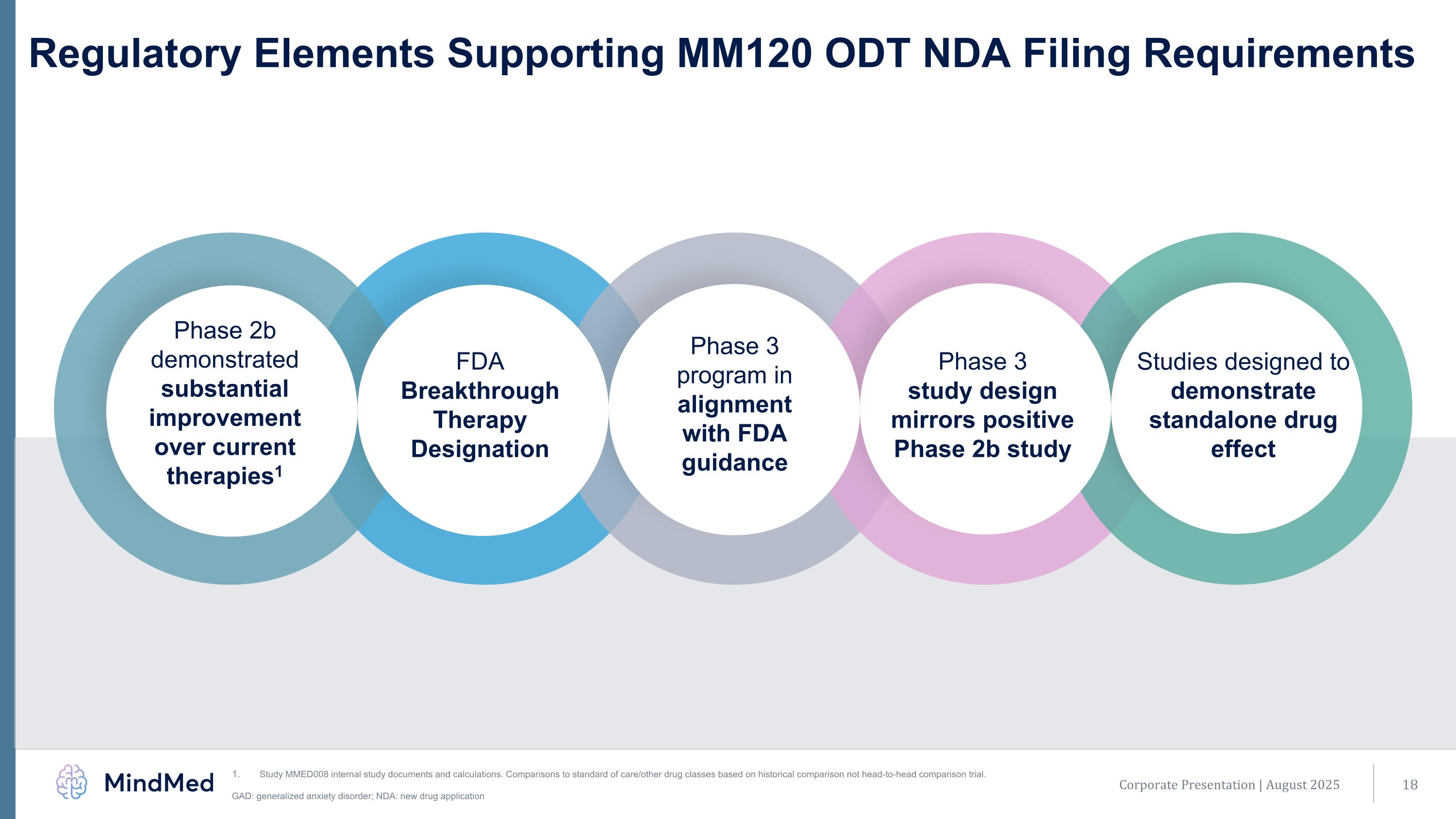
Today. Regulatory Elements Supporting MM120 ODT NDA Filing Requirements Studies designed to demonstrate standalone drug effect FDA Breakthrough Therapy Designation Phase 2b demonstrated substantial improvement over current therapies1 Phase 3 program in alignment with FDA guidance Phase 3 study design mirrors positive Phase 2b study Corporate Presentation | August 2025 Study MMED008 internal study documents and calculations. Comparisons to standard of care/other drug classes based on historical comparison not head-to-head comparison trial. GAD: generalized anxiety disorder; NDA: new drug application

Commercial Framework MM120 ODT LSD D-tartrate
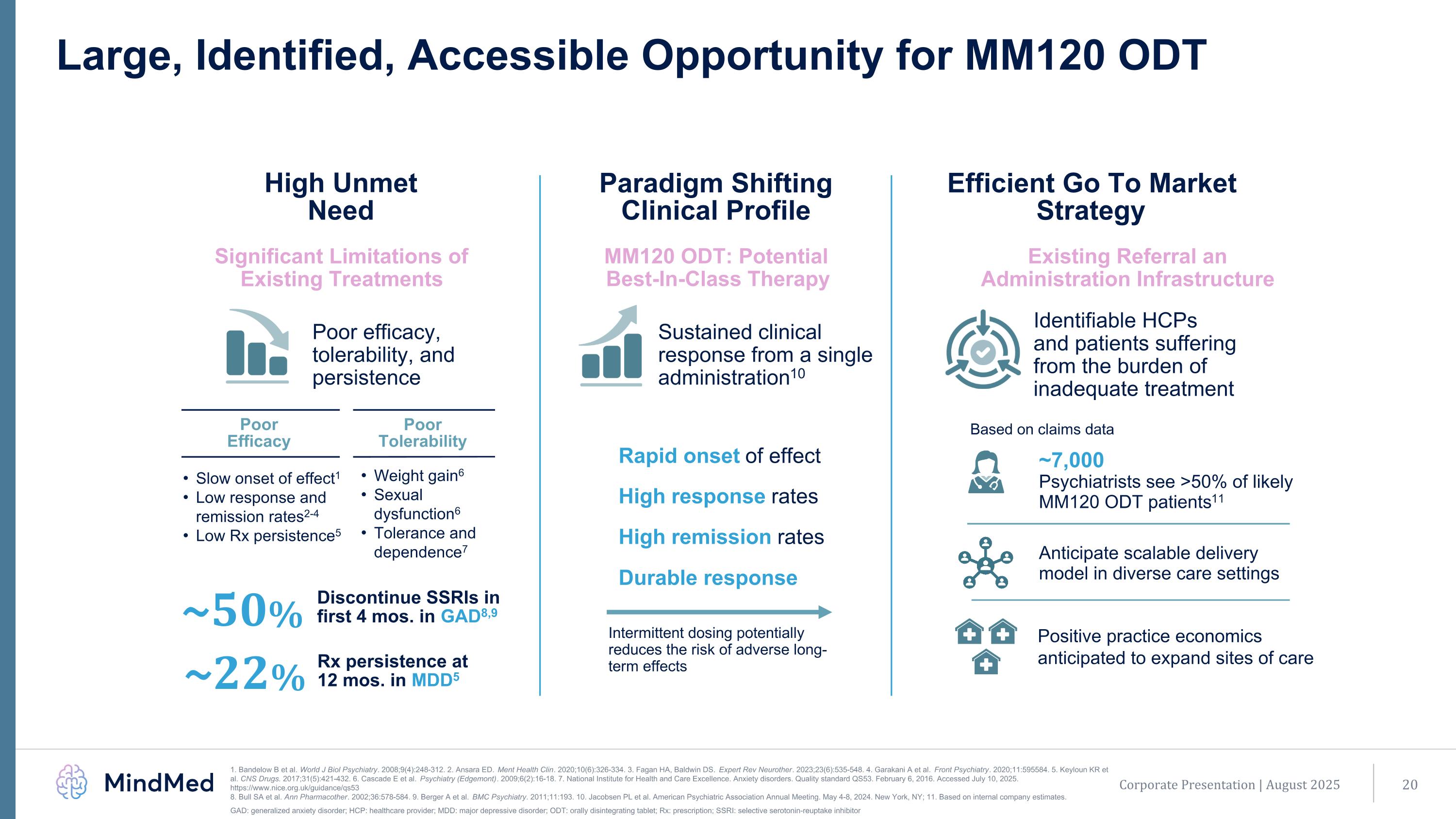
Large, Identified, Accessible Opportunity for MM120 ODT High Unmet Need Paradigm Shifting Clinical Profile Efficient Go To Market Strategy Significant Limitations of Existing Treatments MM120 ODT: Potential Best-In-Class Therapy Existing Referral an Administration Infrastructure Sustained clinical response from a single administration10 Identifiable HCPs and patients suffering from the burden of inadequate treatment Poor efficacy, tolerability, and persistence ~50% Discontinue SSRIs in first 4 mos. in GAD8,9 Rapid onset of effect High response rates High remission rates Durable response Intermittent dosing potentially reduces the risk of adverse long-term effects Slow onset of effect1 Low response and remission rates2-4 Low Rx persistence5 Weight gain6 Sexual dysfunction6 Tolerance and dependence7 Poor Tolerability Poor Efficacy ~7,000 Psychiatrists see >50% of likely MM120 ODT patients11 ~22% Rx persistence at 12 mos. in MDD5 Based on claims data Corporate Presentation | August 2025 1. Bandelow B et al. World J Biol Psychiatry. 2008;9(4):248-312. 2. Ansara ED. Ment Health Clin. 2020;10(6):326-334. 3. Fagan HA, Baldwin DS. Expert Rev Neurother. 2023;23(6):535-548. 4. Garakani A et al. Front Psychiatry. 2020;11:595584. 5. Keyloun KR et al. CNS Drugs. 2017;31(5):421-432. 6. Cascade E et al. Psychiatry (Edgemont). 2009;6(2):16-18. 7. National Institute for Health and Care Excellence. Anxiety disorders. Quality standard QS53. February 6, 2016. Accessed July 10, 2025. https://www.nice.org.uk/guidance/qs53 8. Bull SA et al. Ann Pharmacother. 2002;36:578-584. 9. Berger A et al. BMC Psychiatry. 2011;11:193. 10. Jacobsen PL et al. American Psychiatric Association Annual Meeting. May 4-8, 2024. New York, NY; 11. Based on internal company estimates. GAD: generalized anxiety disorder; HCP: healthcare provider; MDD: major depressive disorder; ODT: orally disintegrating tablet; Rx: prescription; SSRI: selective serotonin-reuptake inhibitor Anticipate scalable delivery model in diverse care settings Positive practice economics anticipated to expand sites of care
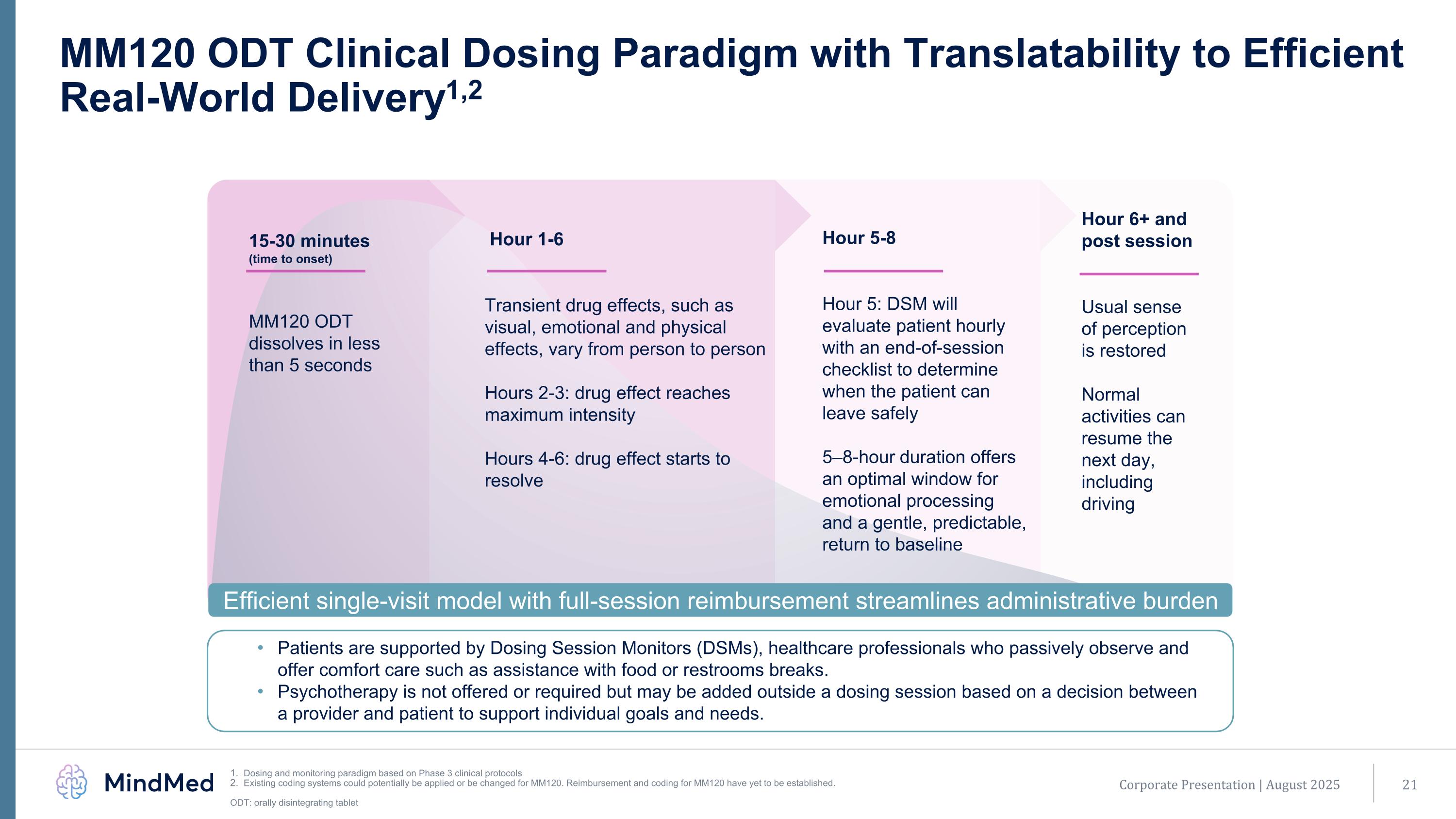
MM120 ODT Clinical Dosing Paradigm with Translatability to Efficient Real-World Delivery1,2 Patients are supported by Dosing Session Monitors (DSMs), healthcare professionals who passively observe and offer comfort care such as assistance with food or restrooms breaks. Psychotherapy is not offered or required but may be added outside a dosing session based on a decision between a provider and patient to support individual goals and needs. Hour 6+ and post session Usual sense of perception is restored Normal activities can resume the next day, including driving Hour 1-6 Transient drug effects, such as visual, emotional and physical effects, vary from person to person Hours 2-3: drug effect reaches maximum intensity Hours 4-6: drug effect starts to resolve 15-30 minutes (time to onset) MM120 ODT dissolves in less than 5 seconds Hour 5-8 Hour 5: DSM will evaluate patient hourly with an end-of-session checklist to determine when the patient can leave safely 5–8-hour duration offers an optimal window for emotional processing and a gentle, predictable, return to baseline Efficient single-visit model with full-session reimbursement streamlines administrative burden Corporate Presentation | August 2025 Dosing and monitoring paradigm based on Phase 3 clinical protocols Existing coding systems could potentially be applied or be changed for MM120. Reimbursement and coding for MM120 have yet to be established. ODT: orally disintegrating tablet
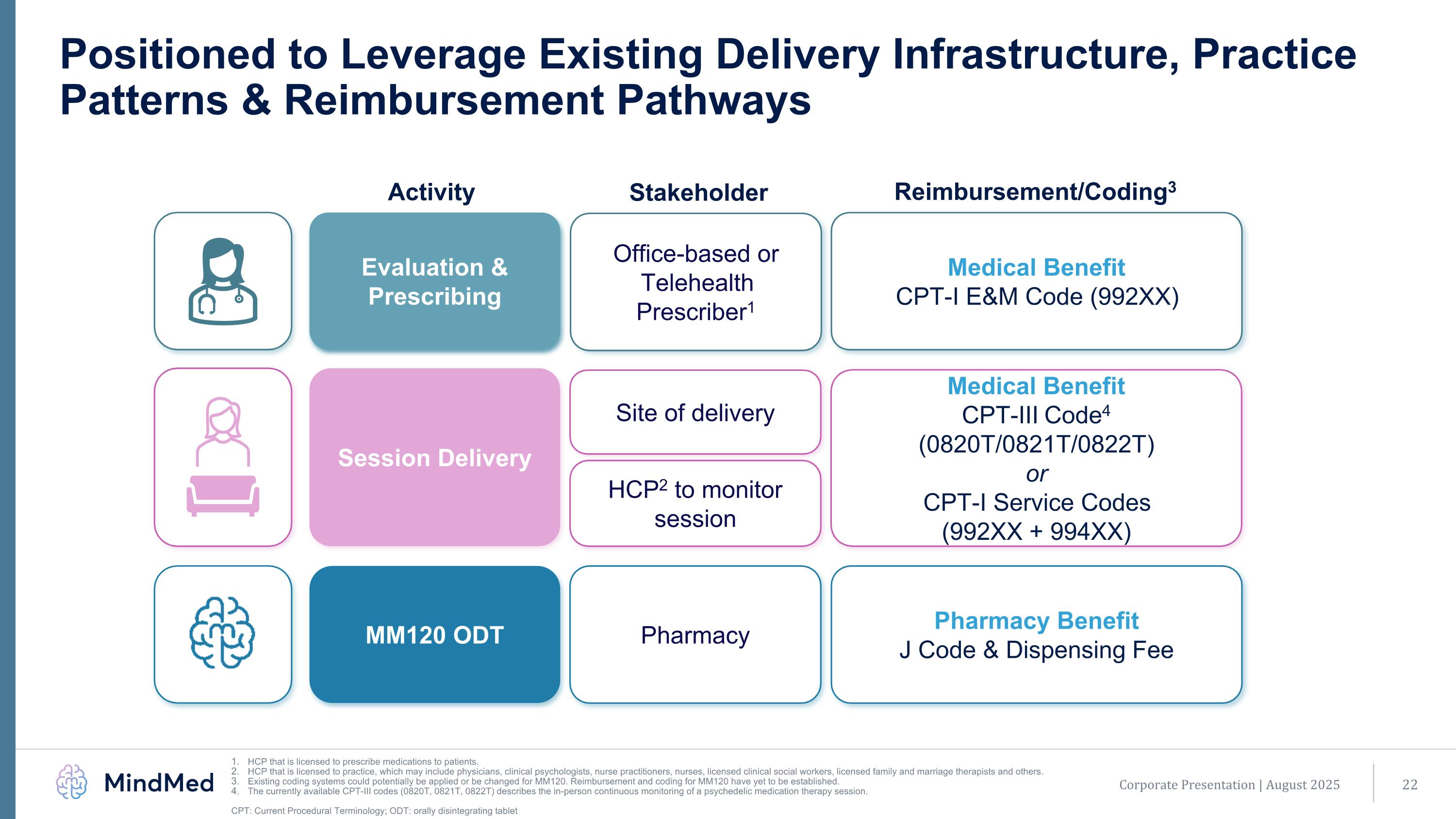
Corporate Presentation | August 2025 Positioned to Leverage Existing Delivery Infrastructure, Practice Patterns & Reimbursement Pathways HCP that is licensed to prescribe medications to patients. HCP that is licensed to practice, which may include physicians, clinical psychologists, nurse practitioners, nurses, licensed clinical social workers, licensed family and marriage therapists and others. Existing coding systems could potentially be applied or be changed for MM120. Reimbursement and coding for MM120 have yet to be established. The currently available CPT-III codes (0820T, 0821T, 0822T) describes the in-person continuous monitoring of a psychedelic medication therapy session. CPT: Current Procedural Terminology; ODT: orally disintegrating tablet Office-based or Telehealth Prescriber1 Evaluation & Prescribing Pharmacy MM120 ODT Site of delivery Session Delivery Activity Medical Benefit CPT-I E&M Code (992XX) Pharmacy Benefit J Code & Dispensing Fee Medical Benefit CPT-III Code4 (0820T/0821T/0822T) or CPT-I Service Codes (992XX + 994XX) Stakeholder Reimbursement/Coding3 HCP2 to monitor session
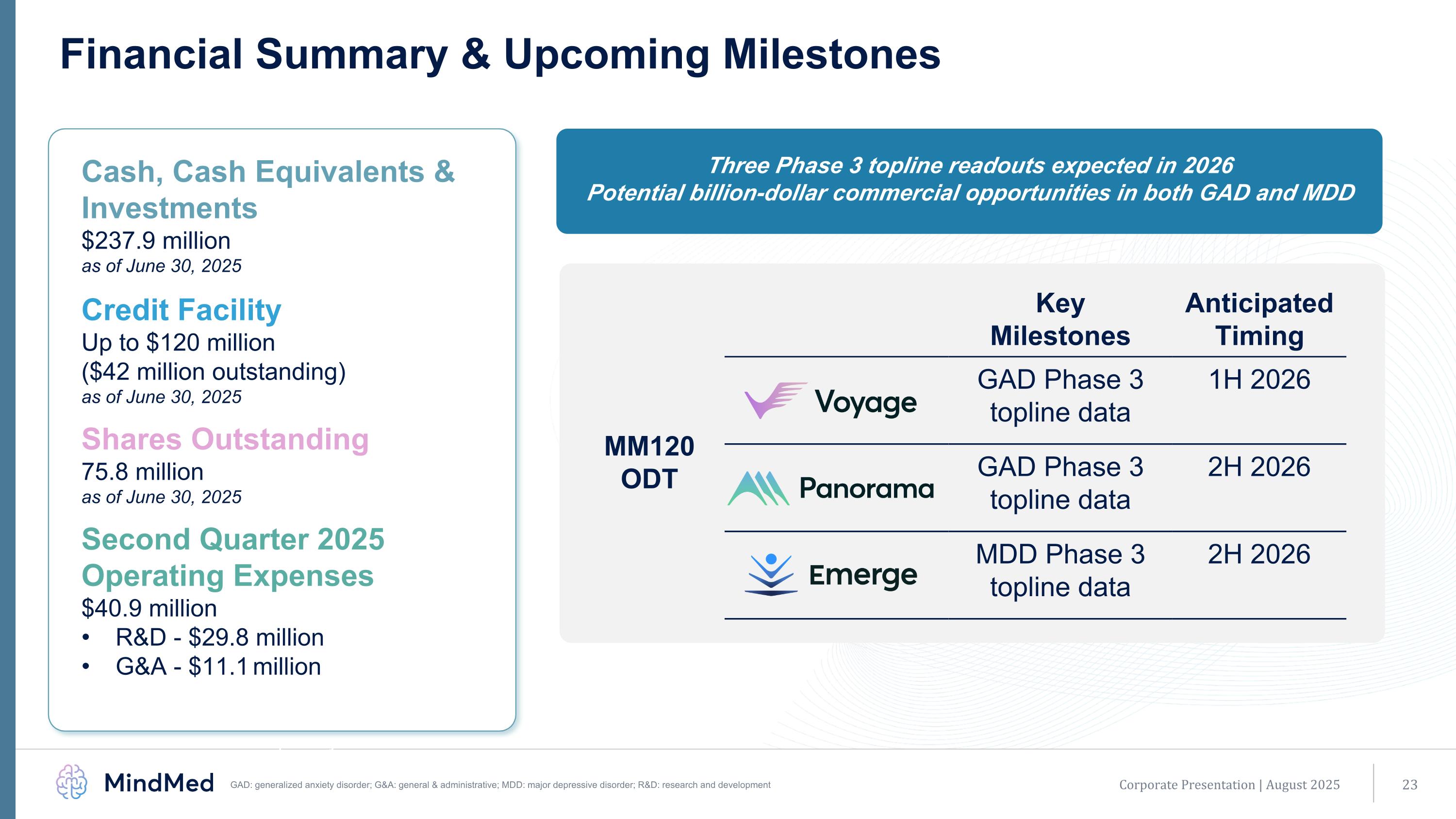
$250 million in equity investment Initiation of Phase 3 program for MM120 ODT in GAD (first patient dosed in Phase 3 Voyage study) MM120 Phase 2b results presented at APA Annual Meeting MM120 granted breakthrough designation by U.S. FDA Successful end-of-phase 2 meeting with U.S. FDA supporting pivotal trial plans MM120 ODT patent issued covering pharmaceutical formulation, methods of manufacturing and treatment; patent life through 2041 MM120 ODT awarded Innovation Passport by the U.K. MHRA Financial Summary & Upcoming Milestones Corporate Presentation | August 2025 Key Milestones Anticipated Timing GAD Phase 3 topline data 1H 2026 GAD Phase 3 topline data 2H 2026 MDD Phase 3 topline data 2H 2026 Three Phase 3 topline readouts expected in 2026 Potential billion-dollar commercial opportunities in both GAD and MDD Cash, Cash Equivalents & Investments $237.9 million as of June 30, 2025 Credit Facility Up to $120 million ($42 million outstanding) as of June 30, 2025 Shares Outstanding 75.8 million as of June 30, 2025 Second Quarter 2025 Operating Expenses $40.9 million R&D - $29.8 million G&A - $11.1 million MM120 ODT GAD: generalized anxiety disorder; G&A: general & administrative; MDD: major depressive disorder; R&D: research and development

Nasdaq: MNMD

Appendix
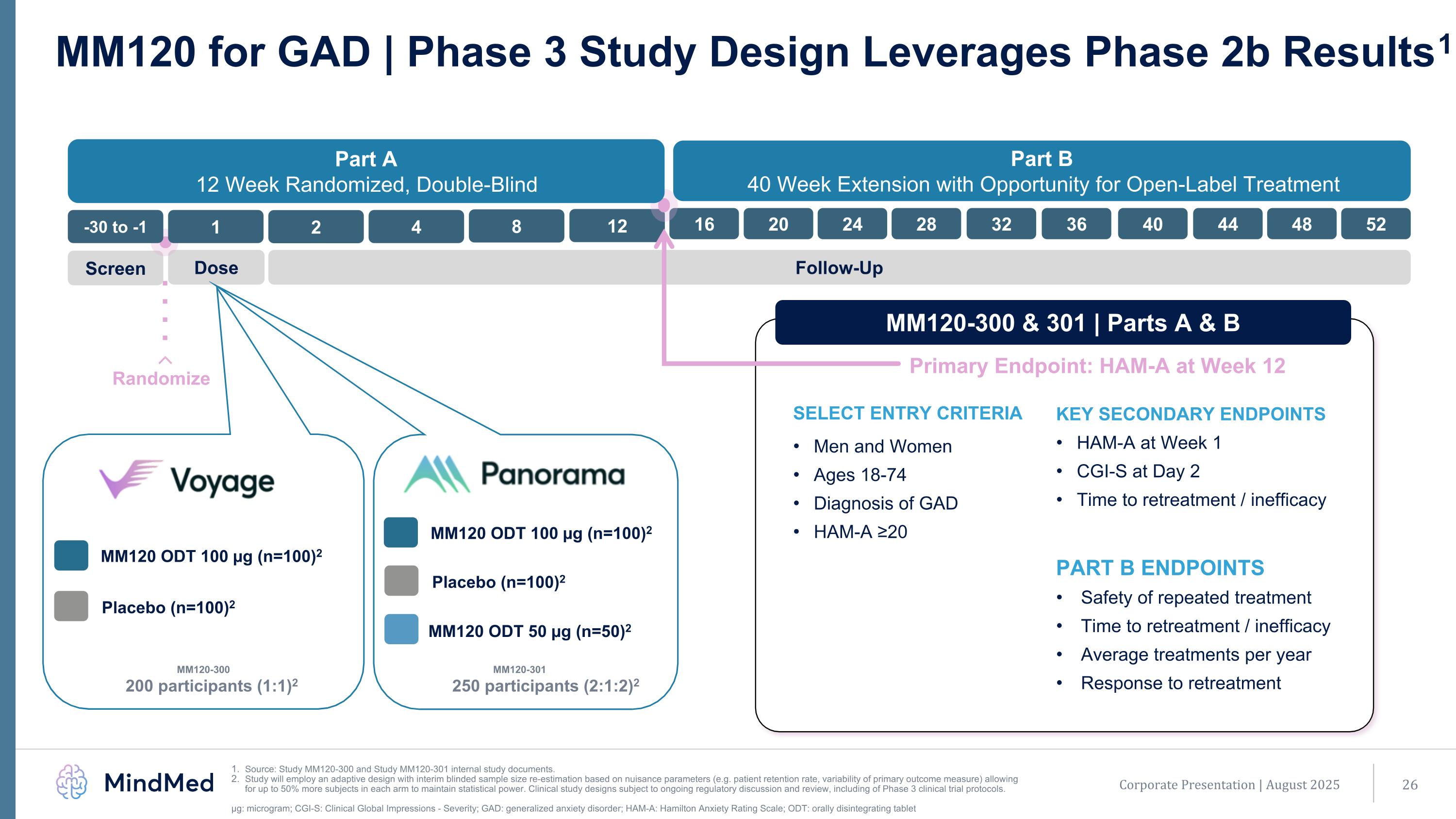
MM120-301 250 participants (2:1:2)2 Randomize Screen Dose Follow-Up MM120 ODT 100 µg (n=100)2 MM120 ODT 50 µg (n=50)2 Placebo (n=100)2 MM120-300 200 participants (1:1)2 MM120 ODT 100 µg (n=100)2 Placebo (n=100)2 -30 to -1 1 2 4 8 12 16 20 24 28 32 36 40 44 48 52 Corporate Presentation | August 2025 MM120 for GAD | Phase 3 Study Design Leverages Phase 2b Results1 Source: Study MM120-300 and Study MM120-301 internal study documents. Study will employ an adaptive design with interim blinded sample size re-estimation based on nuisance parameters (e.g. patient retention rate, variability of primary outcome measure) allowing for up to 50% more subjects in each arm to maintain statistical power. Clinical study designs subject to ongoing regulatory discussion and review, including of Phase 3 clinical trial protocols. μg: microgram; CGI-S: Clinical Global Impressions - Severity; GAD: generalized anxiety disorder; HAM-A: Hamilton Anxiety Rating Scale; ODT: orally disintegrating tablet KEY SECONDARY ENDPOINTS HAM-A at Week 1 CGI-S at Day 2 Time to retreatment / inefficacy SELECT ENTRY CRITERIA Men and Women Ages 18-74 Diagnosis of GAD HAM-A ≥20 Part A 12 Week Randomized, Double-Blind Part B 40 Week Extension with Opportunity for Open-Label Treatment Primary Endpoint: HAM-A at Week 12 PART B ENDPOINTS Safety of repeated treatment Time to retreatment / inefficacy Average treatments per year Response to retreatment MM120-300 & 301 | Parts A & B
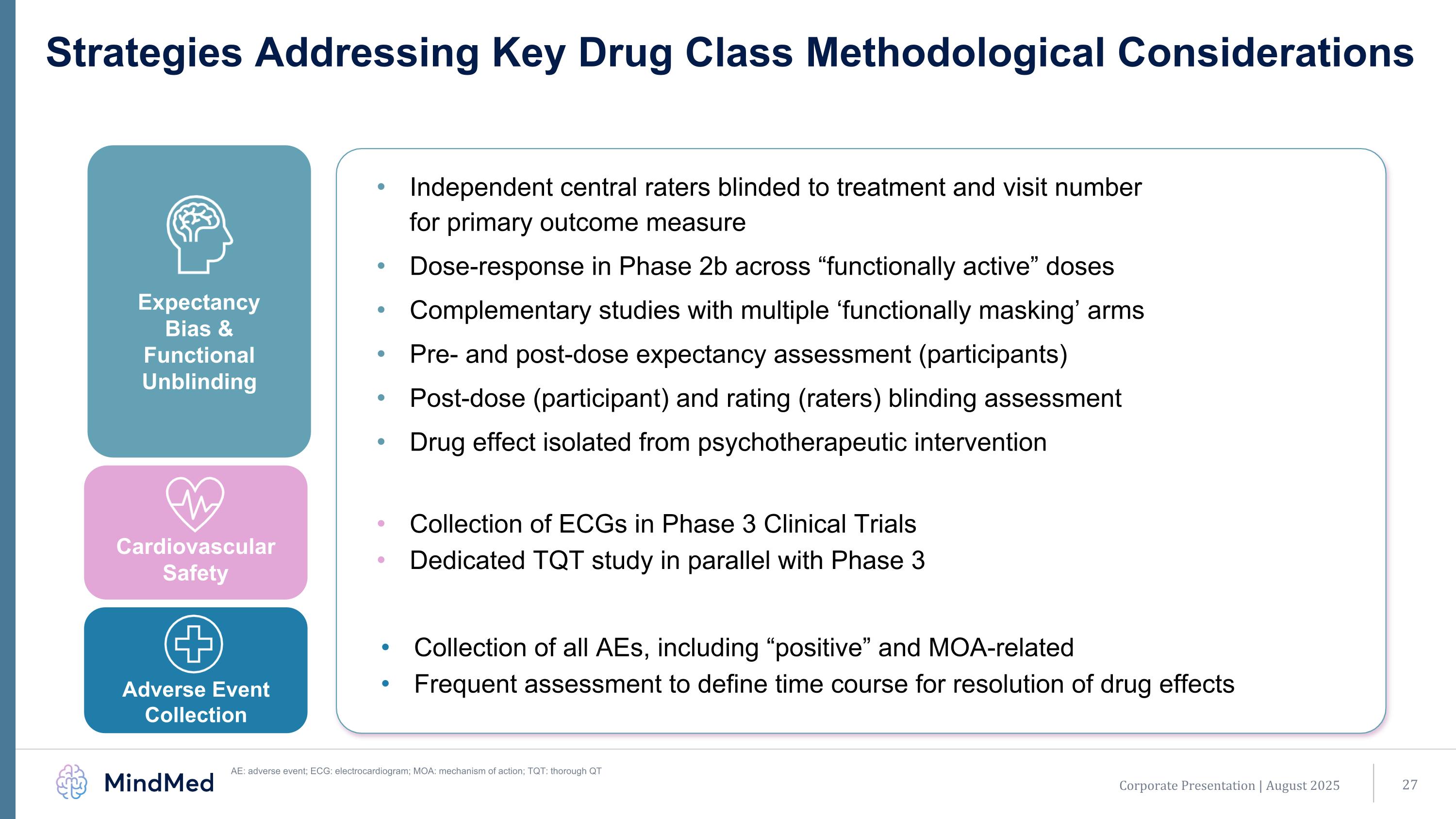
Strategies Addressing Key Drug Class Methodological Considerations AE: adverse event; ECG: electrocardiogram; MOA: mechanism of action; TQT: thorough QT Expectancy Bias & Functional Unblinding Cardiovascular Safety Adverse Event Collection Independent central raters blinded to treatment and visit number for primary outcome measure Dose-response in Phase 2b across “functionally active” doses Complementary studies with multiple ‘functionally masking’ arms Pre- and post-dose expectancy assessment (participants) Post-dose (participant) and rating (raters) blinding assessment Drug effect isolated from psychotherapeutic intervention Collection of ECGs in Phase 3 Clinical Trials Dedicated TQT study in parallel with Phase 3 Collection of all AEs, including “positive” and MOA-related Frequent assessment to define time course for resolution of drug effects Corporate Presentation | August 2025
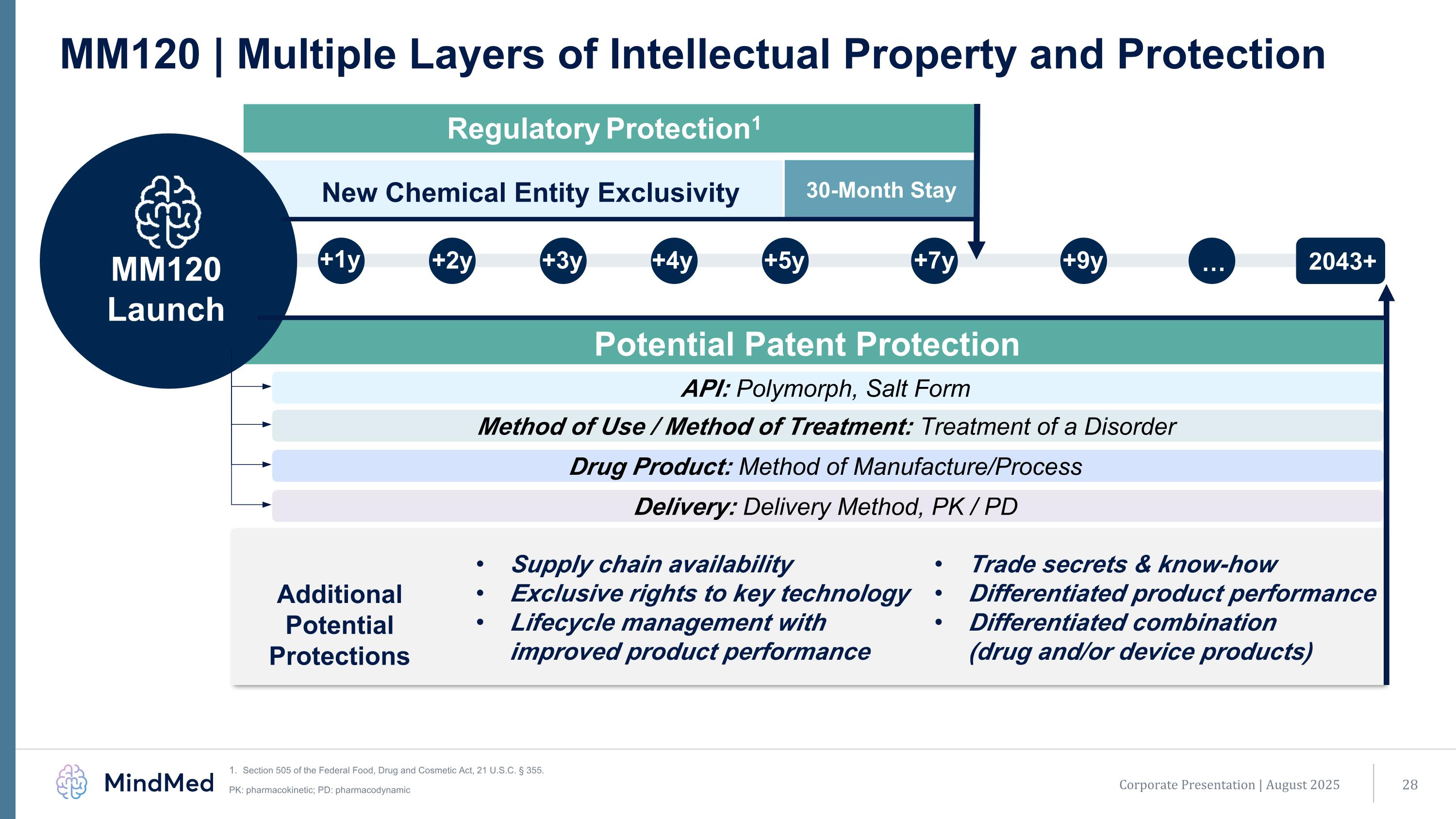
+1y +2y +3y +4y +9y +5y +7y … 2043+ New Chemical Entity Exclusivity 30-Month Stay MM120 | Multiple Layers of Intellectual Property and Protection Section 505 of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 355. PK: pharmacokinetic; PD: pharmacodynamic MM120 Launch Potential Patent Protection Method of Use / Method of Treatment: Treatment of a Disorder Delivery: Delivery Method, PK / PD Drug Product: Method of Manufacture/Process API: Polymorph, Salt Form Supply chain availability Exclusive rights to key technology Lifecycle management with improved product performance Trade secrets & know-how Differentiated product performance Differentiated combination (drug and/or device products) Additional Potential Protections Regulatory Protection1 Corporate Presentation | August 2025