
Exhibit 99.1

Company Logo MindMed Investor Presentation August 2023

Disclaimer This presentation (the “Presentation”) has been prepared by Mind Medicine (MindMed) Inc. (“MindMed” or the “Company”) solely for informational purposes. None of MindMed, its affiliates or any of their respective employees, directors, officers, contractors, advisors, members, successors, representatives or agents makes any representation or warranty as to the accuracy or completeness of any information contained in this Presentation and shall have no liability for any representations (expressed or implied) contained in, or for any omissions from, this Presentation. This presentation shall not constitute an offer, nor a solicitation of an offer, of the sale or purchase of securities. This Presentation does not constitute an offering of securities of MindMed and under no circumstances is it to be construed as a prospectus or advertisement or public offering of securities. Any trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of the products or services of MindMed. Any amounts are in USD unless otherwise noted. MindMed’s securities have not been approved or disapproved by the SEC or by any state, provincial or other securities regulatory authority, nor has the SEC or any state, provincial or other securities regulatory authority passed on the accuracy or adequacy of this Presentation. Any representation to the contrary is a criminal offense. Cautionary Note Regarding Forward-Looking Statements This Presentation contains, and our officers and representatives may from time to time make, “forward-looking statements” within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995 and other applicable securities laws. Forward-looking statements can often, but not always, be identified by words such as “plans”, “expects”, “is expected”, “budget”, “scheduled”, “estimates”, “forecasts”, “intends”, “anticipates”, will”, “projects”, or “believes” or variations (including negative variations) of such words and phrases, or statements that certain actions, events, results or conditions “may”, “could”, “would”, “might” or “will” be taken, occur or be achieved, and similar references to future periods. Except for statements of historical fact, examples of forward-looking statements include, among others, statements pertaining to the development and commercialization of any medicine or treatment, or the efficacy of either of the foregoing, the success and timing of our development activities, the success and timing of our planned clinical trials, our ability to meet the milestones set forth herein; the likelihood of success of any clinical trials or of obtaining FDA or other regulatory approvals, the likelihood of obtaining patents or the efficacy of such patents once granted, and the potential for the markets that MindMed is anticipating to access. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the economy and other future conditions as of the date of this Presentation. While we consider these assumptions to be reasonable, the assumptions are inherently subject to significant business, social, economic, political, regulatory, competitive and other risks and uncertainties that are difficult to predict and many of which are outside of our control, and our actual results and financial condition may differ materially from those indicated in the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause our actual results and financial condition to differ materially from those indicated in the forward-looking statements include, among others, the following: our ability to raise capital to complete its plans and fund its studies; the medical and commercial viability of the contemplated medicines and treatments being developed; our ability to raise additional capital in the future as we continue to develop our products; our history of negative cash flows; our limited operating history; incurrence of future losses; lack of revenue; compliance with laws and regulations; difficulty associated with research and development; risks associated with clinical trials or studies; heightened regulatory scrutiny; early stage product development; clinical trial risks; regulatory approval processes; novelty of the psychedelic inspired medicines industry; as well as those risk factors discussed or referred to throughout the “Risk Factors” sections of our Annual Report on Form 10-K for the year ended December 31, 2022 and our Quarterly Report on Form 10-Q for the quarter ended June 30, 2023 filed with the Securities and Exchange Commission (the “SEC”) and in other filings we make in the future with the SEC and the securities regulatory authorities in all provinces and territories of Canada, available under the Company’s profile on SEDAR at www.sedar.com. Any forward-looking statement made by us in this Presentation is based only on information currently available to us and speaks only as of the date on which it is made. MindMed undertakes no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise. Cautionary Note Regarding Regulatory Matters The United States federal government regulates drugs through the Controlled Substances Act. The Company works with a non-hallucinogenic synthetic derivative of the psychedelic substance ibogaine, known as zolunicant which is a synthetic organic molecule designed around a common coronaridine chemical backbone. Zolunicant is not a Schedule I substance in the United States and the Company does not foresee it becoming a Schedule I substance due to its non-hallucinogenic properties. While the Company is focused on programs using psychedelic or hallucinogenic compounds and non-hallucinogenic derivatives of these compounds, the Company does not have any direct or indirect involvement with the illegal selling, production or distribution of any substances in the jurisdictions in which it operates. The Company is a neuro-pharmaceutical drug development company and does not deal with psychedelic or hallucinogenic substances except within laboratory and clinical trial settings conducted within approved regulatory frameworks. The Company’s products will not be commercialized prior to applicable regulatory approval, which will only be granted if clinical evidence of safety and efficacy for the intended uses is successfully developed. Market and Industry Data This Presentation includes market and industry data that has been obtained from third party sources, including industry publications. MindMed believes that the industry data is accurate and that the estimates and assumptions are reasonable, but there is no assurance as to the accuracy or completeness of this data. Third party sources generally state that the information contained therein has been obtained from sources believed to be reliable, but there is no assurance as to the accuracy or completeness of included information. Although the data is believed to be reliable, MindMed has not independently verified any of the data from third party sources referred to in this Presentation or ascertained the underlying economic assumptions relied upon by such sources. References in this Presentation to research reports or to articles and publications should be not construed as depicting the complete findings of the entire referenced report or article. MindMed does not make any representation as to the accuracy of such information. Investor Presentation | August 2023 2
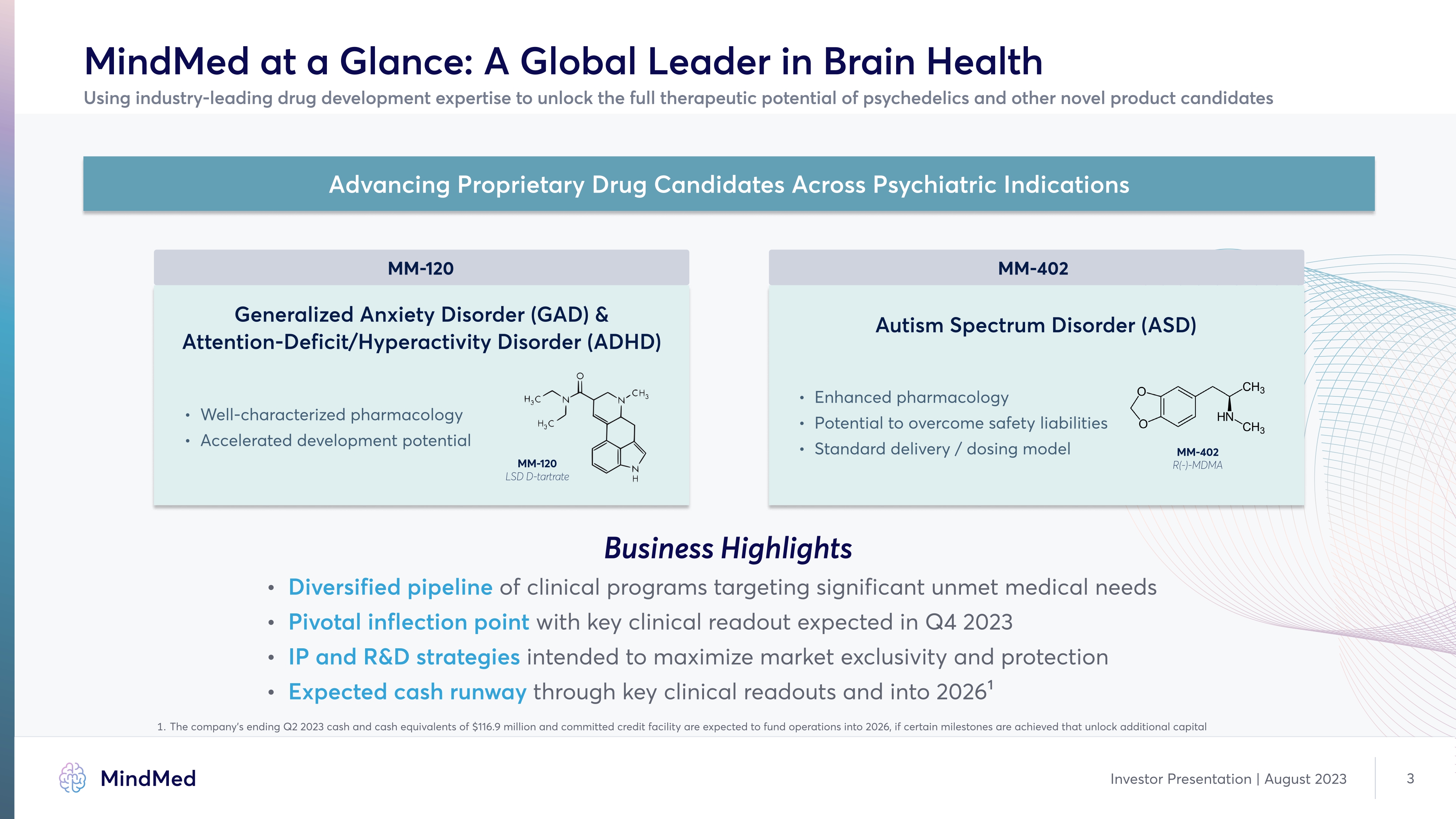
MindMed at a Glance: A Global Leader in Brain Health Using industry-leading drug development expertise to unlock the full therapeutic potential of psychedelics and other novel product candidates MM-120 Generalized Anxiety Disorder (GAD) & Attention-Deficit/Hyperactivity Disorder (ADHD) Well-characterized pharmacolog Accelerated development potential MM-120 LSD D-tartrate MM-402 Autism Spectrum Disorder (ASD) Enhanced pharmacolog Potential to overcome safety liabilitie Standard delivery / dosing model MM-402 R(-)-MDMA Business Highlights Diversified pipeline of clinical programs targeting significant unmet medical need Pivotal inflection point with key clinical readout expected in Q4 2023 IP and R&D strategies intended to maximize market exclusivity and protection Expected cash runway through key clinical readouts and into 2026 1 1 The company s ending Q2 2023 cash and cash equivalents of $116.9 million and committed credit facility are expected to fund operations into 2026, if certain milestones are achieved that unlock additional capital Company logo MindMed Investor Presentation | August 2023 3
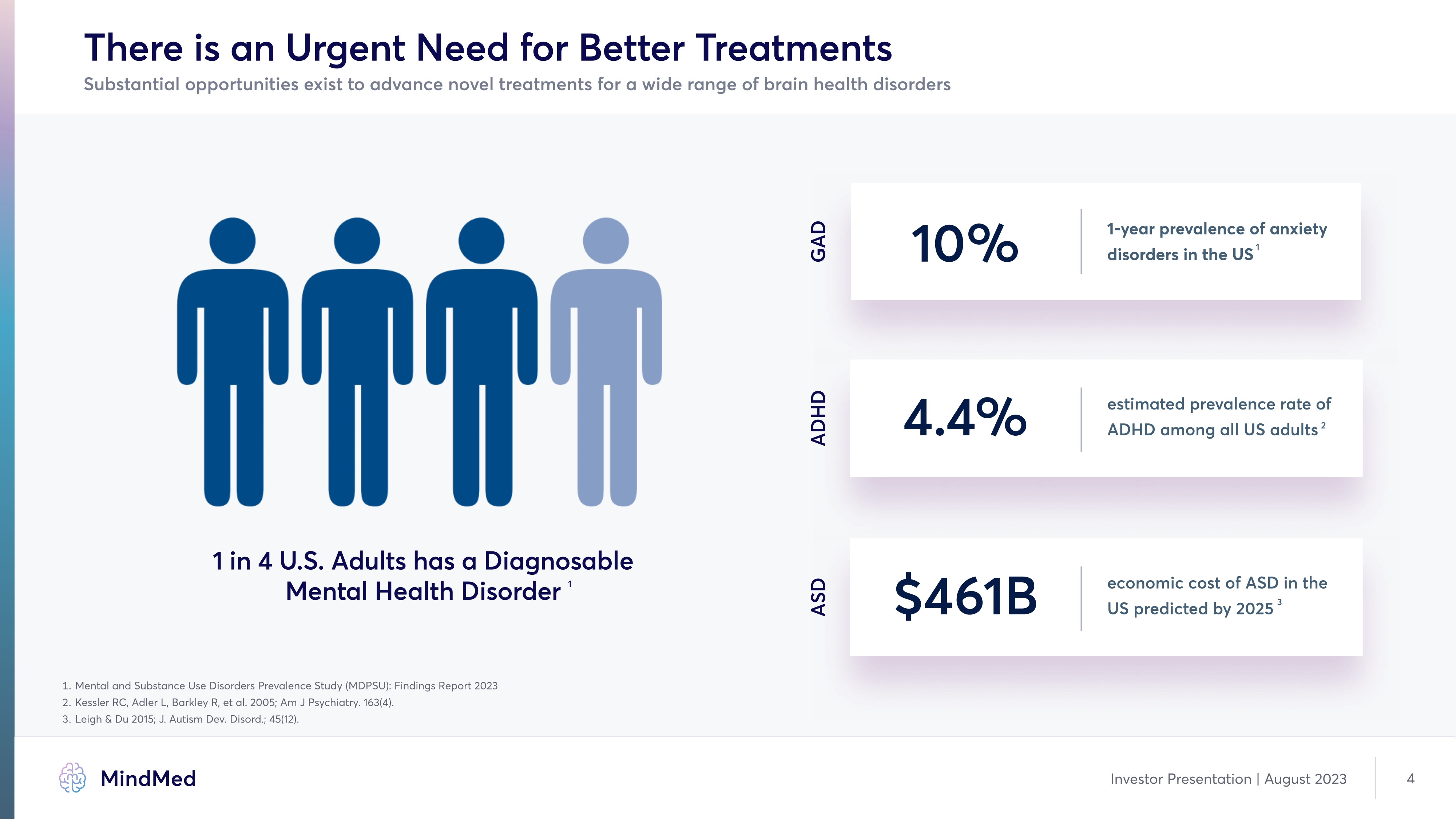
There is an Urgent Need for Better Treatments Substantial opportunities exist to advance novel treatments for a wide range of brain health disorders GAD 10% 1-year prevalence of anxiety disorders in the US1 ADHD 4.4% estimated prevalence rate of ADHD among all US adults 2 ASD $461B economic cost of ASD in the US predicted by 2025 3 1 in 4 U.S. Adults has a Diagnosable Mental Health Disorder GAD 1 1. Mental and Substance Use Disorders Prevalence Study (MDPSU): Findings Report 2023 2. kessler RC, Adler L, BarkleÏ R, et al. 2005; Am J Psychiatry. 163(4). 3. Leigh & Du 2015; J. Autism Dev. Disord.; 45(12). Company Logo Mind Med Investor Presentation | August 2023 4
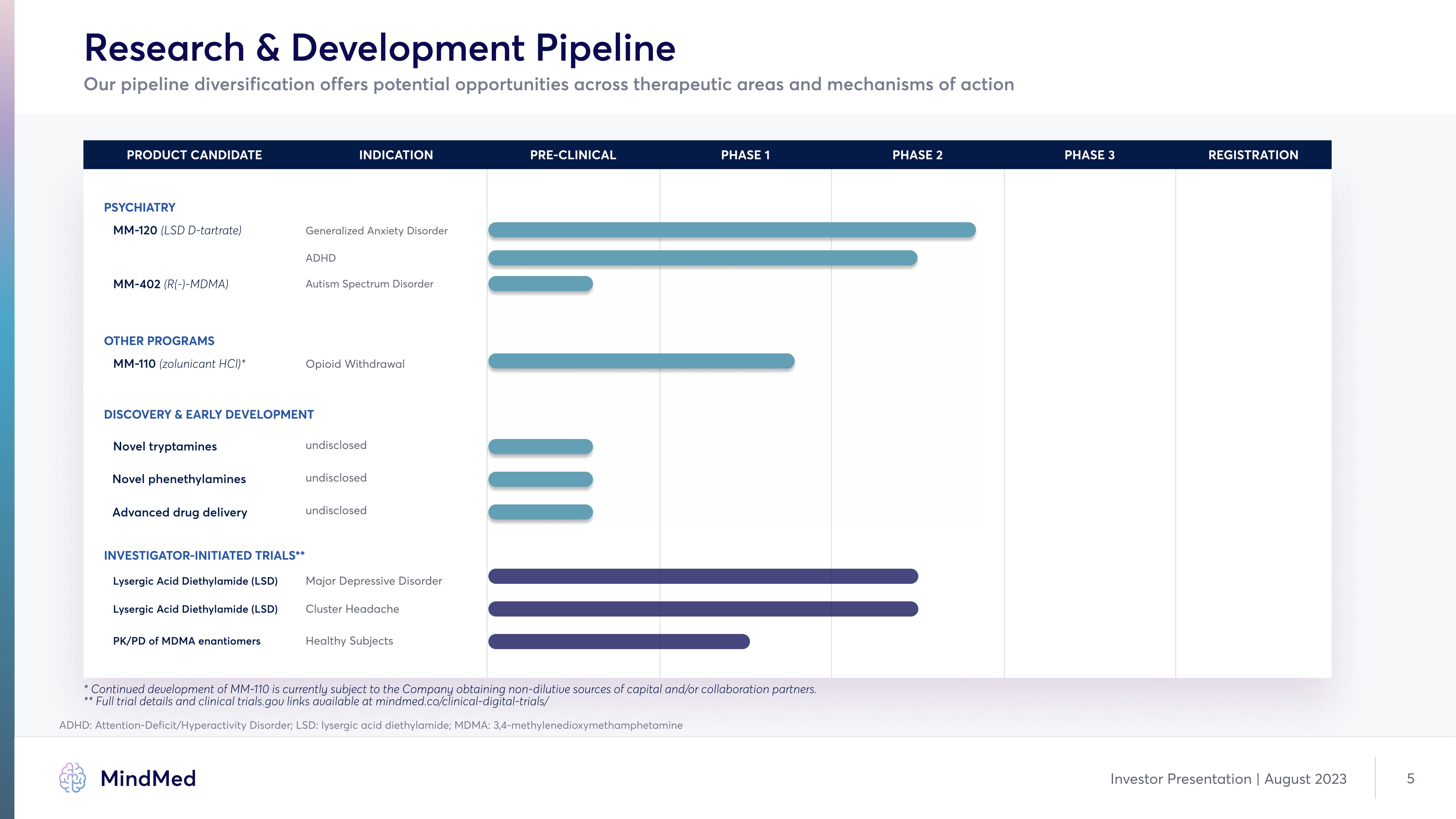
Research & Development Pipeline Our pipeline diversification offers potential opportunities across therapeutic areas and mechanisms of action PRODUCT CANDIDATE INDICATION PRE-CLINICAL PHASE 1 PHASE 2 PHASE 3 REGISTRATION Psychiatry MM-120 (LSD D-tartrate) Generalized Anxiety Disorder ADHD MM-402 (R(-)-MDMA) Autism Spectrum Disorder OTHER PROGRAMS MM-110 (zolunicant HCl)* Opioid Withdrawal DISCOVERY & Early Development Novel tryptamines undisclosed Novel phenethylamines undisclosed Advanced drug delivery undisclosed Investigator-initiated Trials** Lysergic Acid Diethylamide (LSD) Major Depressive Disorder Lysergic Acid Diethylamide (LSD) Cluster Headache PK/PD of MDMA enantiomers Healthy Subjects * Continued development of MM-110 is currently subject to the Company obtaining non-dilutive sources of capital and/or collaboration partners. ** Full trial details and clinical trials.gov links available at mindmed.co/clinical-digital-trials/ ADHD: Attention Deficit/Hyperactivity Disorder; LSD: lysergic acid diethylamide; MDMA: 3,4-methylenedioxymethamphetamine Company Logo MindMed Investor Presentation | August 2023 5
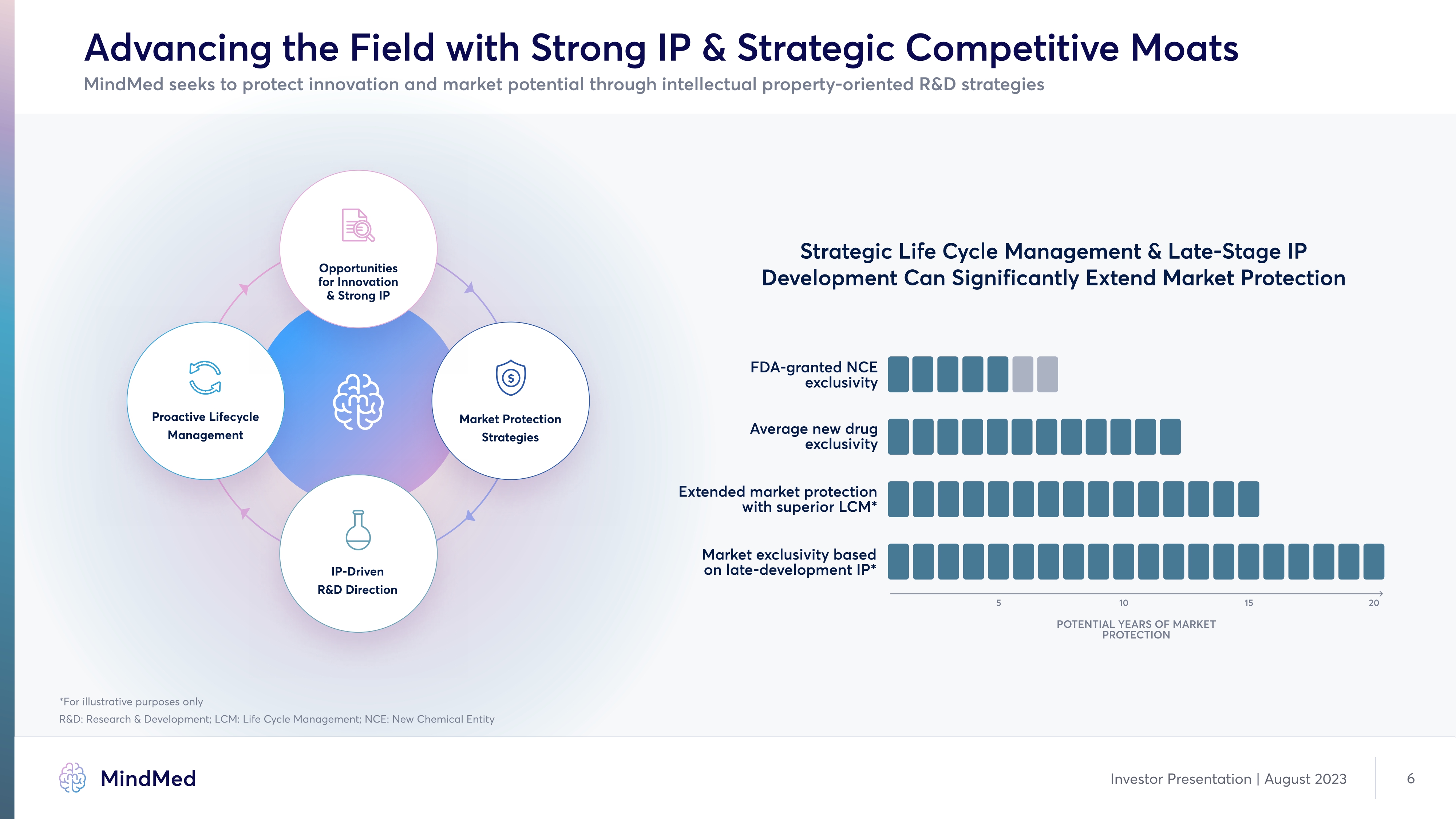
Advancing the Field with Strong IP & Strategic Competitive Moats MindMed seeks to protect innovation and market potential through intellectual property-oriented R&D strategies Strategic Life Cycle Management & Late-Stage IP Development Can Significantly Extend Market Protection FDA-granted NCE exclusivity Average new drug exclusivity Extended market protection with superior LCM* Market exclusivity based on late-development IP* 5 10 15 20 Potential YearS of Market Protection *For illustrative purposes only R&D: Research & Development; LCM: Life Cycle Management; NCE: New Chemical Entity Opportunities for Innovation & Strong IP Proactive Lifecycle Management Market Protection Strategies Market Protection Strategies Diagram Company Logo MindMed Investor Presentation | August 2023 6

Key Milestones Anticipated Phase 2b in GAD Topline Data | Q4 2023 Phase 2a in ADHD Topline Data | Q4 2023 / Q1 2024 MM-120 LSD D-tartrate Company Logo MindMed Investor Presentation | August 2023 7
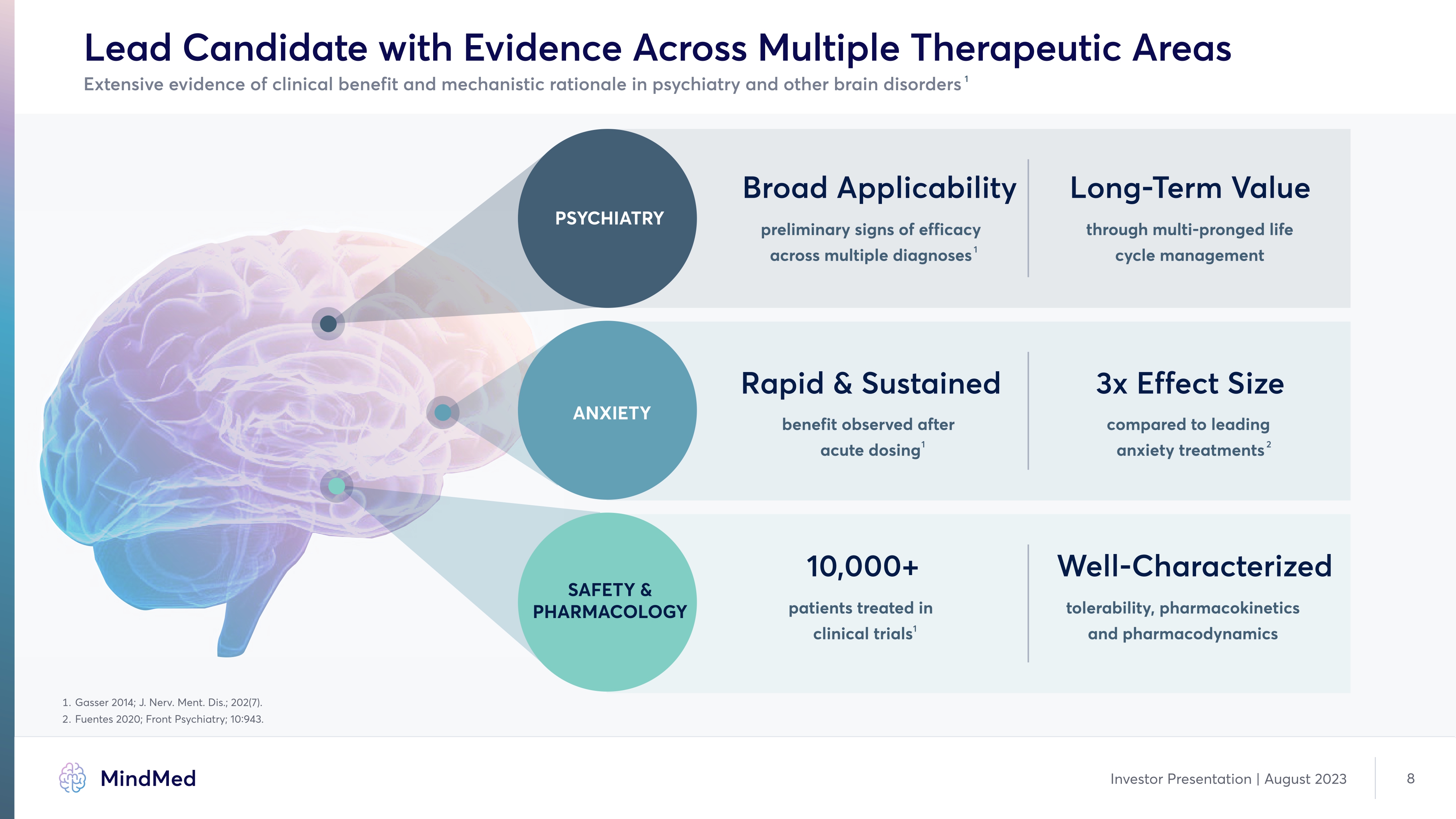
Lead Candidate with Evidence Across Multiple Therapeutic Areas Extensive evidence of clinical benefit and mechanistic rationale in psychiatry and other brain disorders Psychiatry ANXIETY SAFETY & PHARMACOLOGY Broad Applicability preliminary signs of efficacy across multiple diagnoses1 Long-Term Value through multi-pronged life cycle management Rapid & Sustained benefit observed after acute dosing1 3x Effect Size compared to leading anxiety treatments2 10,000+ patients treated in clinical trials1 Well-Characterized tolerability, pharmacokinetics and pharmacodynamics Image 1. Gasser 2014; J. Nerv Ment. Dis.; 202 (7). 2. Fuentes 2020; Front Psychiatry; 10:943. Company Logo MindMed Investor Presentation | August 2023 8
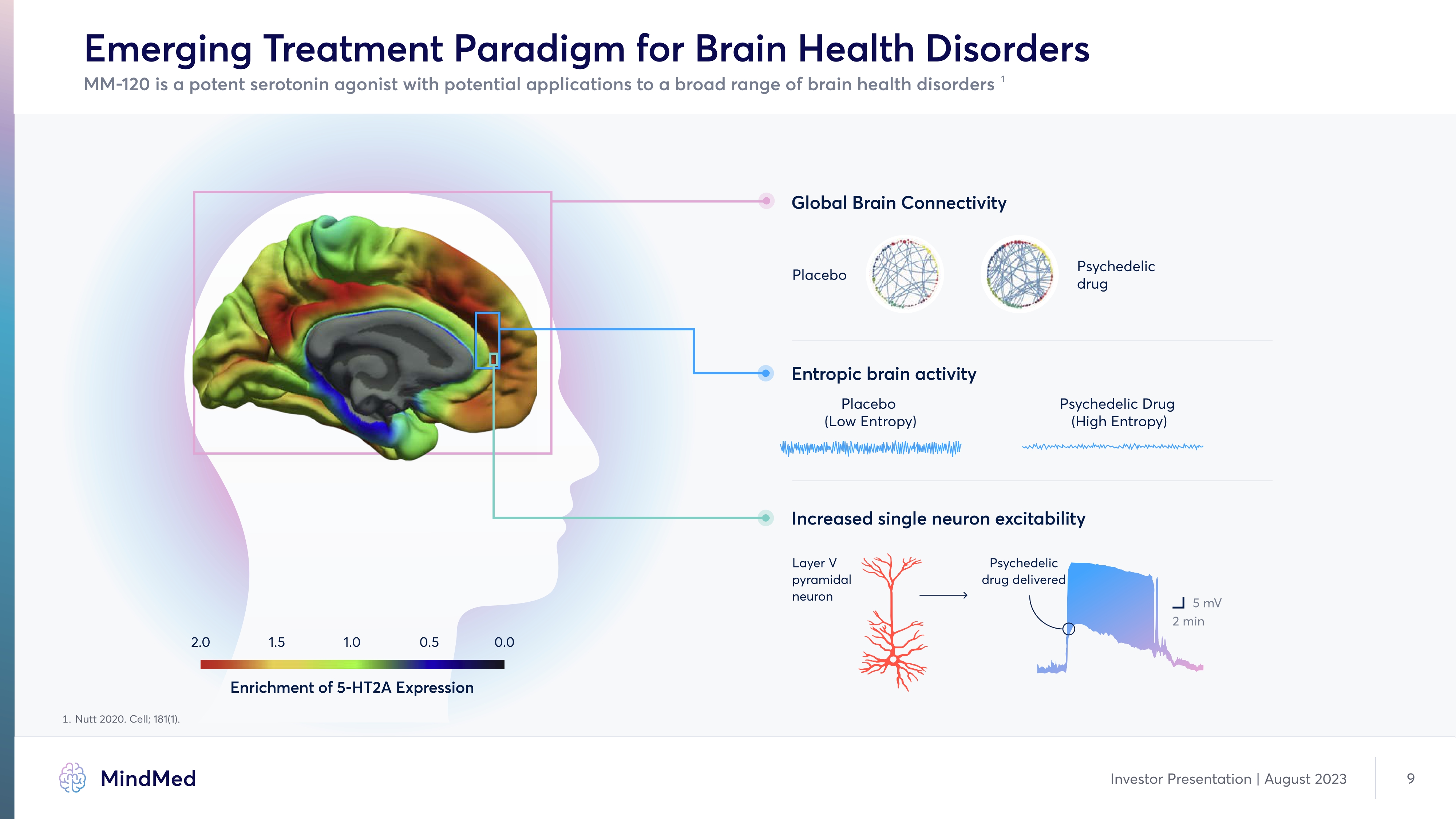
Emerging Treatment Paradigm for Brain Health Disorders Image Image Image Image MM-120 is a potent serotonin agonist with potential applications to a broad range of brain health disorders1 Global Brain Connectivity Placebo Psychedelic drug Entropic brain activity Placebo (Low Entropy) Psychedelic Drug (High Entropy) Increased single neuron excitability Layer V pyramidal neuron Psychedelic drug delivered 5 mV 2 min 2.0 1.5 1.0 0.5 0.0 Enrichment of 5-HT2A Expression 1. Nutt 2020í Cell; 181(1). Company Logo Mind Med Investor Presentation | August 2023 9
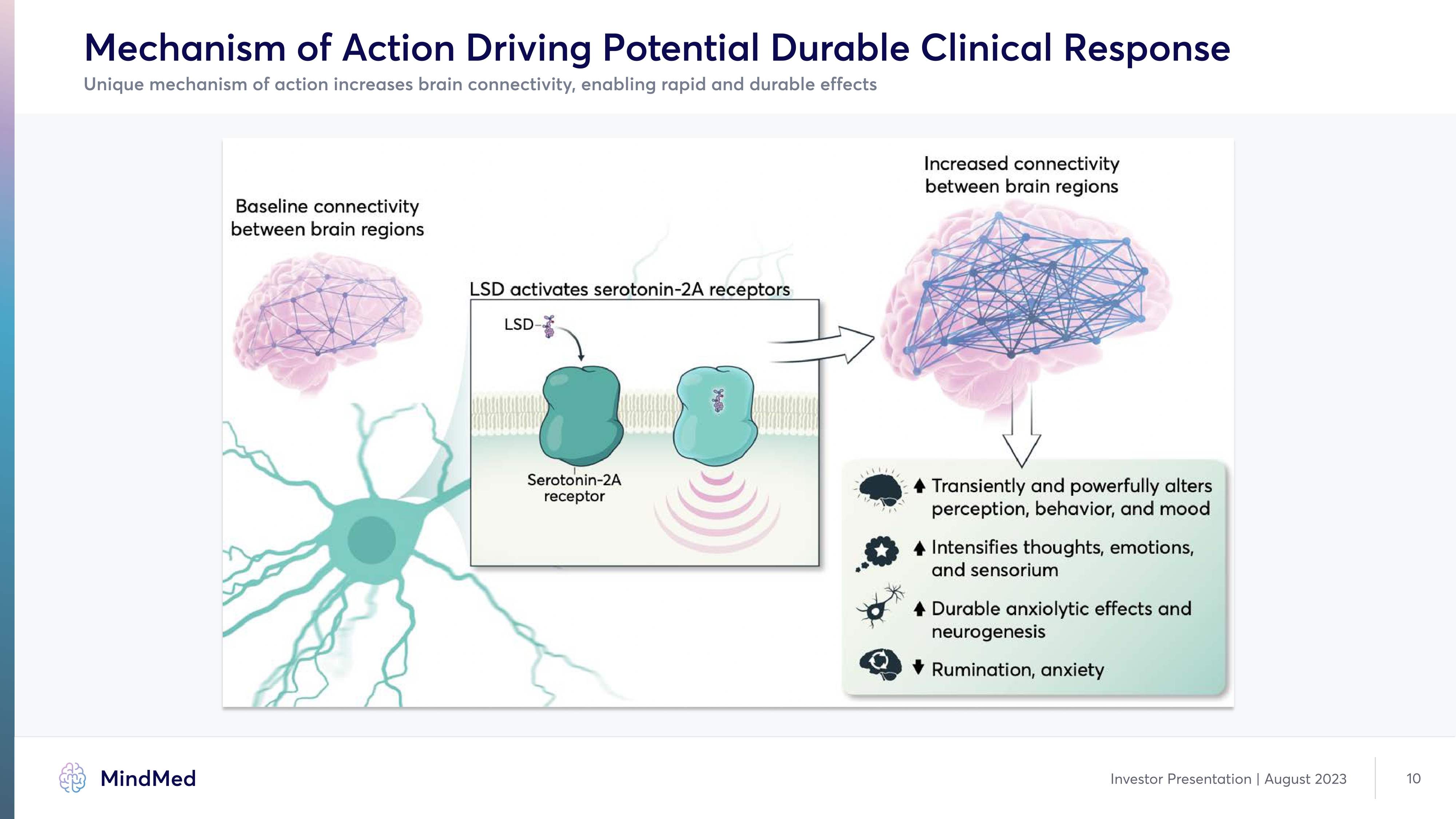
Mechanism of Action Driving Potential Durable Clinical Response Unique mechanism of action increases brain connectivity, enabling rapid and durable effects Increased connectivity between brain regions Baseline connectivity between brain regions LSD activates serotonin-2A receptors LSD Serotonin-2A receptor Transiently and powerfully alters perception, behavior, and mood Intensifies thoughts, emotions, and sensorium Durable anxiolytic effects and neurogenesis Rumination, anxiety Image Image Image Image Image Image Image Image Image Image Company Logo Investor Presentation | August 2023 10
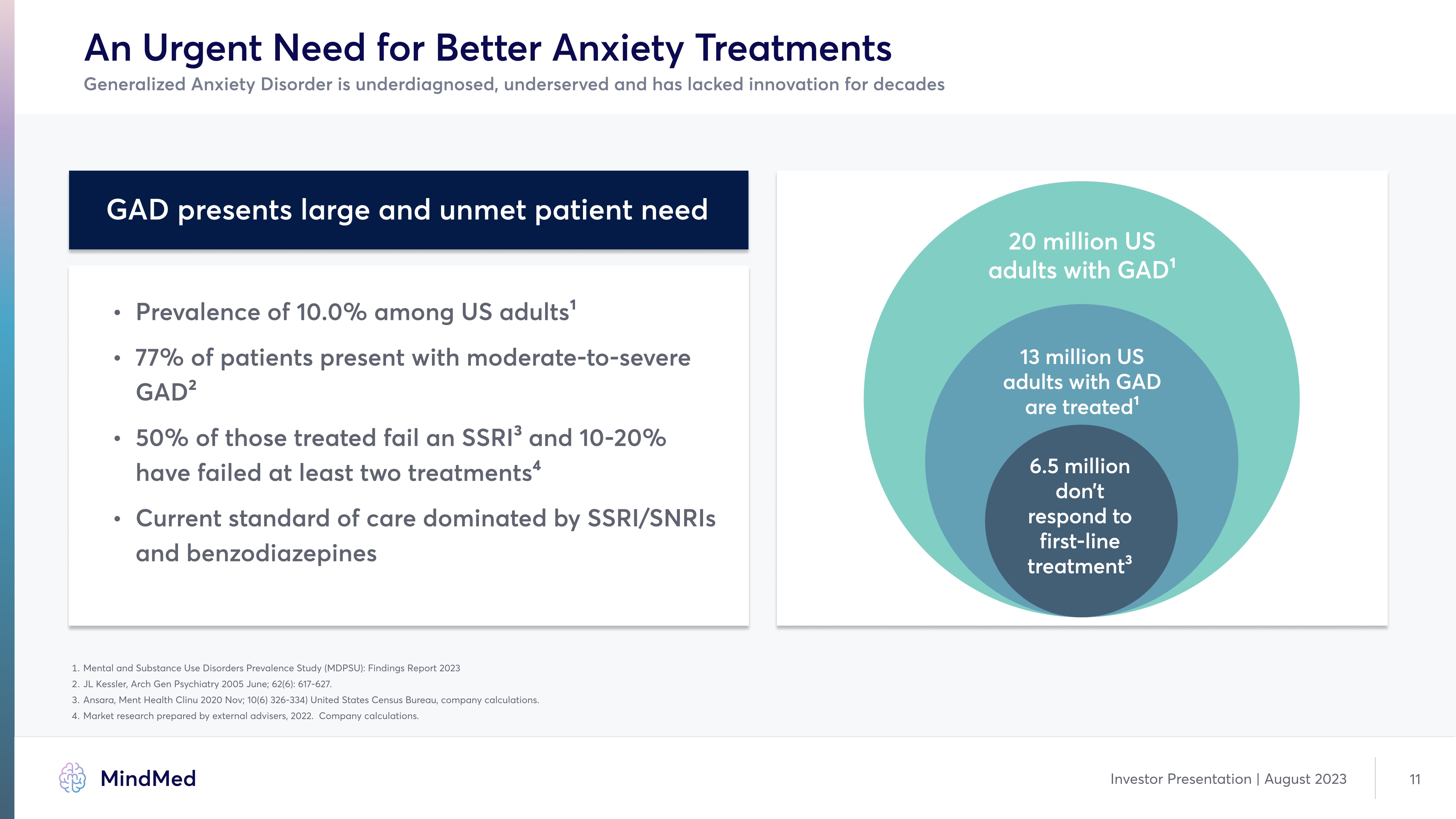
An Urgent Need for Better Anxiety Treatments Generalized Anxiety Disorder is underdiagnosed, underserved and has lacked innovation for decades GAD presents large and unmet patient need Prevalence of 10.0% among US adults1Ù 77% of patients present with moderate-to-severe GAD2Ù 50% of those treated fail an SSRI3 and 10-20% have failed at least two treatments4 Ù Current standard of care dominated by SSRI/SNRIs and benzodiazepines 20 million US adults with GAD1 13 million US adults with GAD are treated1 6.5 million don’t respond to first-line treatment3 1 Mental and Substance Use Disorders Prevalence Study (MDPSU): Findings Report 2023 2 JL kessler, Arch Gen Psychiatry 2005 June; 62(6): 617-627. 3 Ansara, Ment Health Clinu 2020 Nov; 10(6) 326-334) United States Census Bureau, company calculations.4 Market research prepared by external advisers, 2022. Company calculations. Investor Presentation August 2023 11 Company LOGO MindMed

Extensive LSD Clinical Research in Psychiatric Disorders MM-120 program builds on decades of clinical research of LSD, the most studied drug in its class Studies Indication(s) Sample Size KEY FINDINGS Anxiety Major Depressive Disorder 42 patients 61 patients HOLZE 2022 HOLZE 2023 21 Studies PRIOR TO 19741 Anxiety, depression & neurotic illnesses 1 512 patients Up to 95% reduction in symptoms Gasser 2014 Anxiety in terminal illness Effect size of 1.1 with durable reduction in anxiety at 1 year Rapid and durable reduction in symptoms post treatment. Clinical response in 65% of LSD patients vs. 9% in placebo Significant, rapid, durable and beneficial effects, with benefit maintained for up to 16 weeks post treatment (p=0.008) 1 Rucker 2016. J. Psychopharmacol; 30(12). 2 Gasser 2014. J. Nerv. Ment. Dis.; 202(7). 3 Holze, Gasser et. al 2022. Biological Psychiatry. 4 UHB presentation; April 2023. Investor Presentation | August 2023 12 Company LOGO MindMed
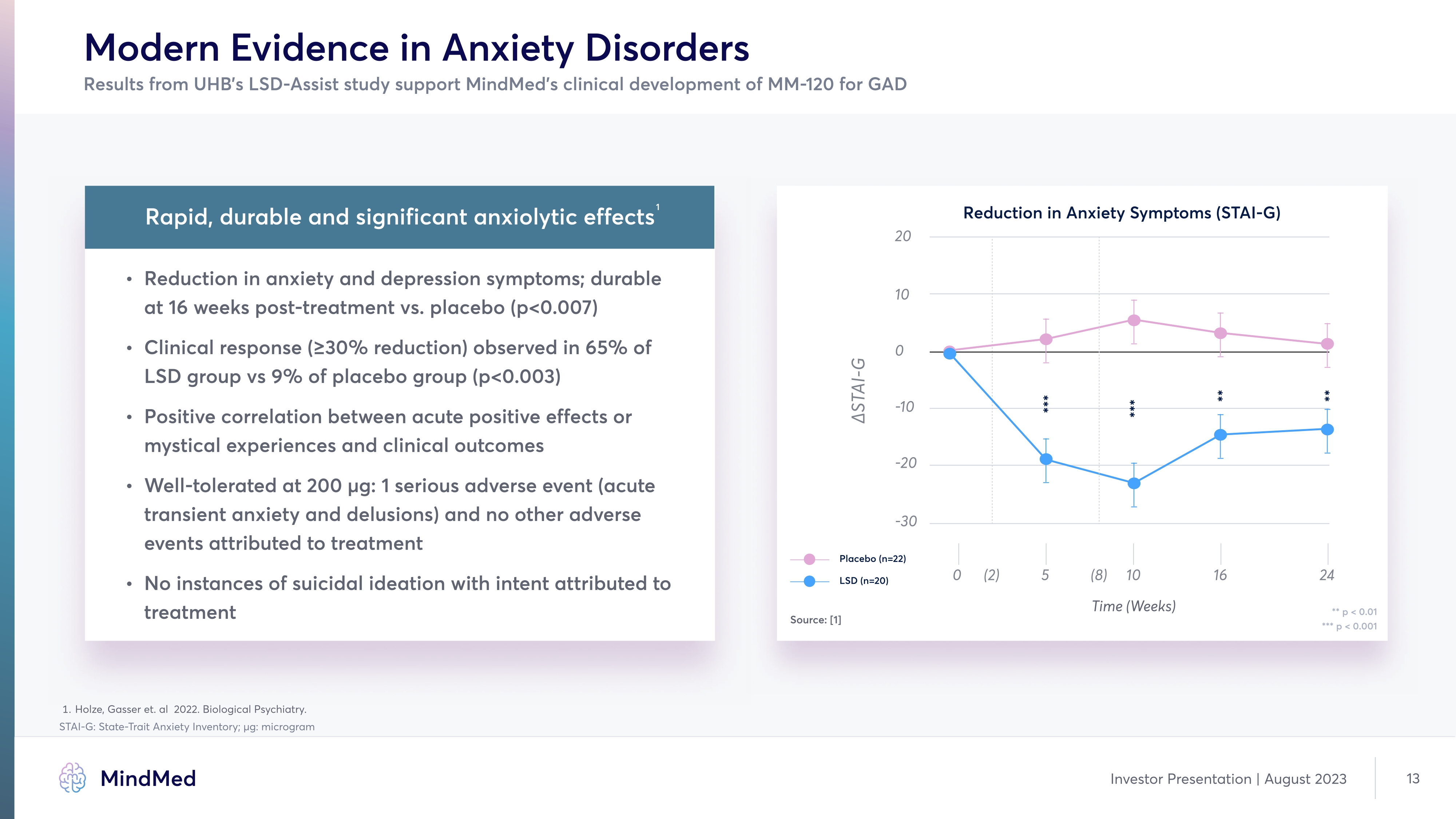
Modern Evidence in Anxiety Disorders Results from UHB’s LSD-Assist study support MindMed’s clinical development of MM-120 for GAD Rapid, durable and significant anxiolytic effects Reduction in anxiety and depression symptoms¬ durable at 16 weeks post-treatment vs. placebo (p<0.007 Clinical response (≥30% reduction observed in 65% of LSD group vs 9% of placebo group (p<0.003 Positive correlation between acute positive effects or mystical experiences and clinical outcome« Well-tolerated at 200 μg: 1 serious adverse event (acute transient anxiety and delusions and no other adverse events attributed to treatment No instances of suicidal ideation with intent attributed to treatment 1 Holze, Gasser et. al 2022. Biological Psychiatry. STAI-G: State-Trait Anxiety Inventory; μg: microgram Investor Presentation August 2023 13 Company LOGO MindMed
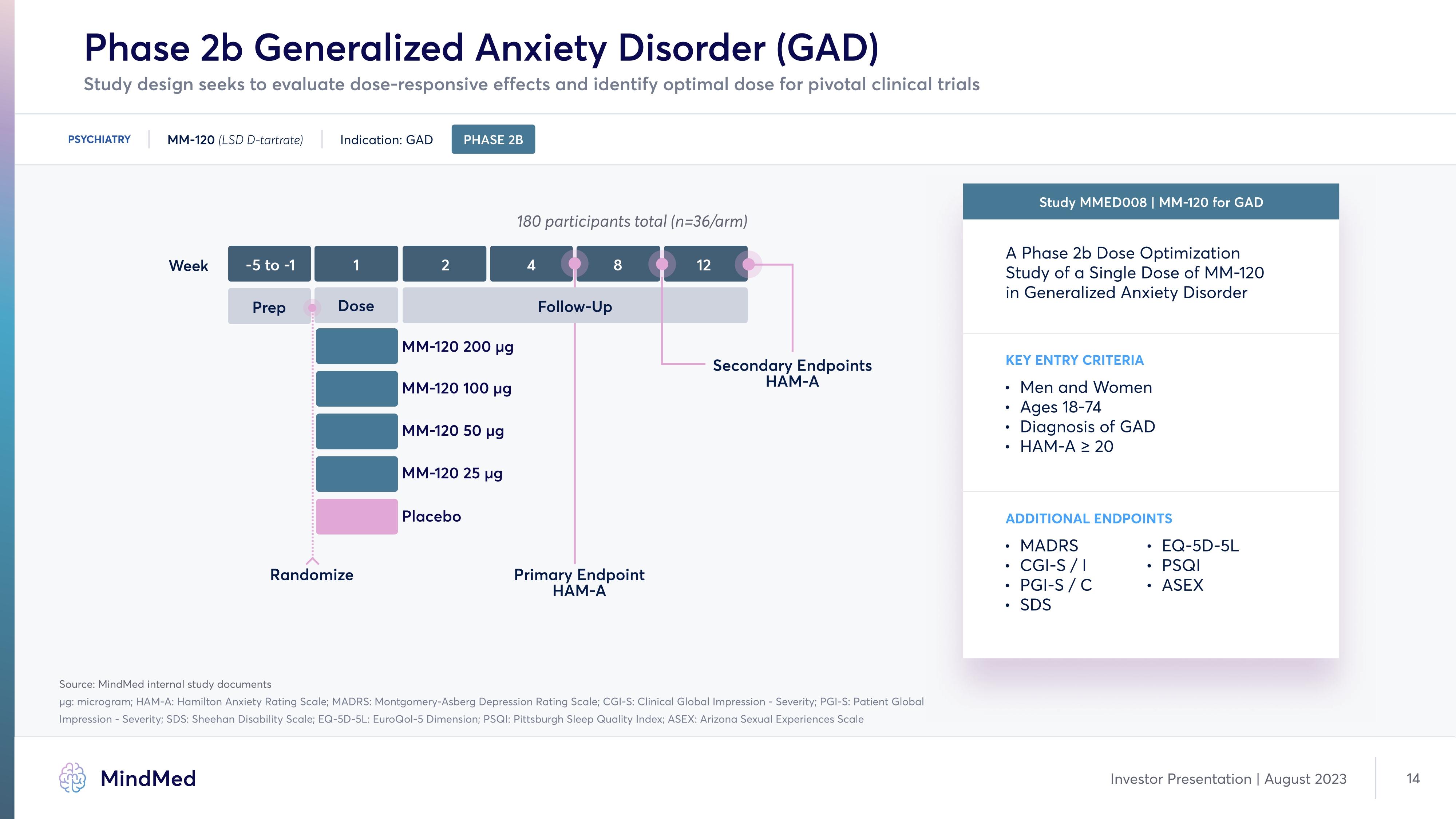
Phase 2b Generalized Anxiety Disorder (GAD) PSYCHIATRY MM-120 (LSD D-tartrate) Indication: GAD PHASE 2B 180 participants total (n=36/arm) ADDITIONAL ENDPOINTS MADRS CGI-S/I PGI-S/C] SDS EQ-5D-5L PSQI ASEX Study MMED008 MM-120 for GAD A Phase 2b Dose Optimization Study of a Single Dose of MM-120 in Generalized Anxiety Disorder Men and Women Ages 18-7È Diagnosis of GAD HAM-A ≥ 20 Key Entry Ëriteria -5 to -1 Prep 4 MM-120 200 µg MM-120 100 µg MM-120 50 µg MM-120 25 µg Placebo 1 Dose 2 8 Primary Endpoint HAM-A Secondary Endpoints HAM-A Week 12 Follow Up Randomize MindMed Investor Presentation August 2023 14 Study design seeks to evaluate dose-responsive effects and identify optimal dose for pivotal clinical trials Company LOGO MindMed

Potential MM-120 Clinical Care Model Advancing a delivery model that seeks to optimize outcomes Pre-Treatment Patient education, engagement, preparation Eligibility evaluation Care coordination with existing clinical team During Treatment Continuous monitoring by qualified session monitors Non-directive psychosocial support Accompanied discharge when release criteria met Post-Treatment Follow-up psychosocial support Continuation of standard psychiatric care Remote monitoring for re-treatment needs Investor Presentation August 2023 15 Company LOGO MindMed

Digital Unlocks Potential Opportunities Throughout the Product Lifecycle Generating data, insights, models, and tools from early development through market management Preclinical Research IND & Phases 1 - 3 Drug Launch Enhancement and Lifecycle Management Clinical Development Tools Patient education, engagement, preparation Deep digital diagnosis Companion Products In-session monitoring predictive intervention Treatment selection Post-Approval Research Surveillance & registries Remote management HEOR Combination Products Drug-device combinations Lifecycle enhancement Efficient phase 4 research HEOR: health economics and outcomes research Investor presentation August 2023 16 Company LOGO MindMed

Potential Pathway to Commercial Success for MM-120 Our approach seeks to leverage well-established pathways to bring novel therapeutics to patients at scale Submit Marketing Applications Seek approval for drug product candidates in major markets globally Collaborate with healthcare authorities to seek targeted labeling Strategic plans for long-term product life cycle management and market preservation Rescheduling Review rescheduling processes of preceding product Advance conversations with national, federal, and state authorities Propose rescheduling in marketing applications Reimbursement Engage payers to develop a comprehensive market access strategy Generate HEOR evidence to maximize reimbursability of drug and dosing session Develop provider tools to enhance reliability of reimbursement Real-World Adoptability Employ a precedent-based development strategy that bridges the novelty of the therapeutic class with the existing care delivery landscape HEOR: health economics outcomes research Investor Presentation August 2023 17 Company LOGO MindMed
Phase 2a Attention-Deficit Hyperactivity Disorder (ADHD) Multi-faceted approach directly targeting the serotonin system Maximizing MM-120 value through study of various doses and schedules to optimize the drug across indications Serotonin is a critical and increasingly in psychiatry Creatively exploring innovative treatment paradigms Repeated sub-perceptual doses of MM-120 in ADHD seek to demonstrate proof of principle for both the regimen and at-home delivery Investor Presentation August 2023 18 Company LOGO MindMed
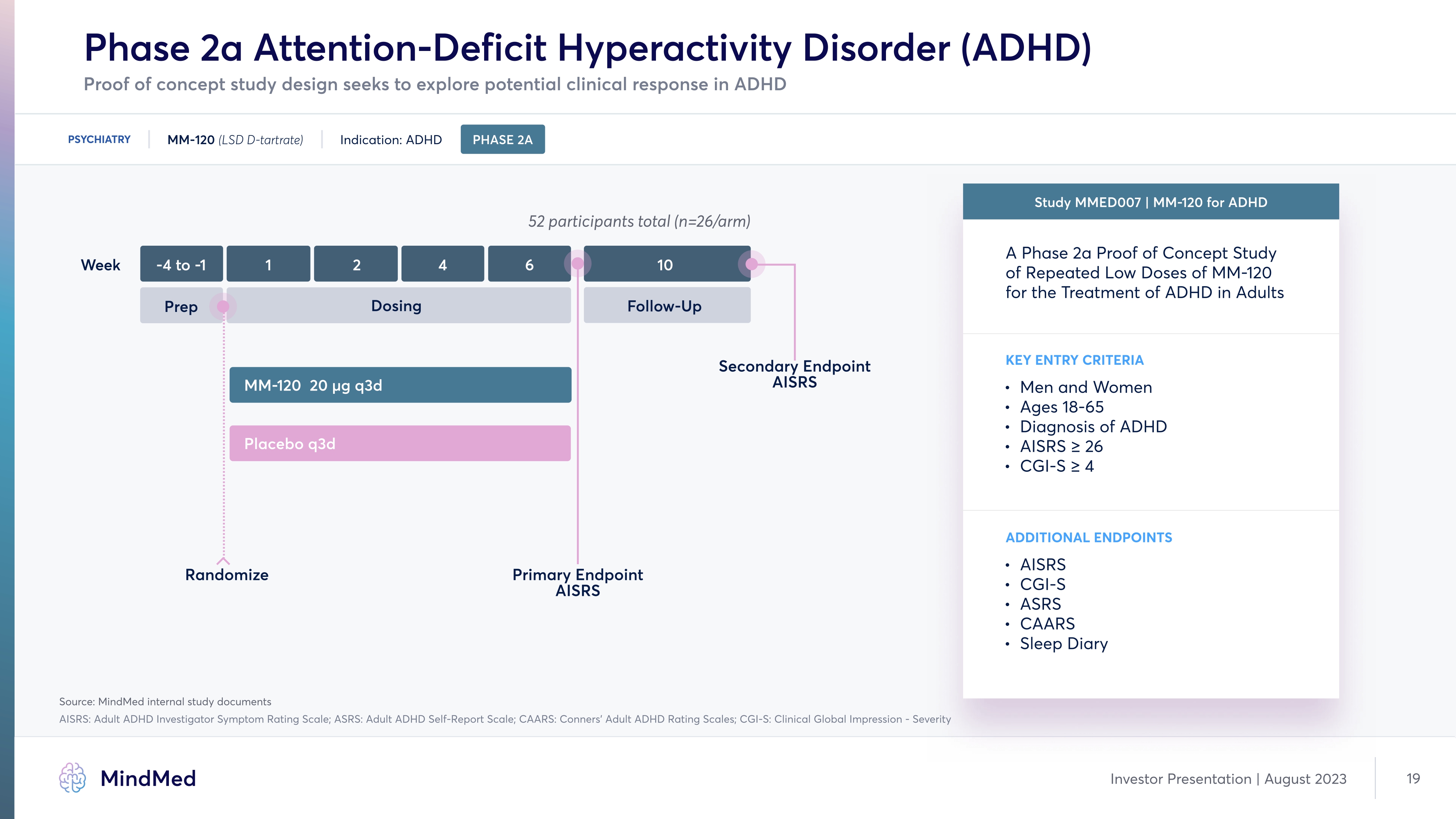
Phase 2a Attention-Deficit Hyperactivity Disorder (ADHD) Proof of concept study design seeks to explore potential clinical response in ADHD PSYCHIATRY MM-120 (LSD D-tartrate) Indication: ADHD PHASE 2A 52 participants total (n=26/arm) Week 2 4 6 10 Prep Dosing Follow Up Secondary Endpoint AISRS MM-120 20 µg q3d Placebo q3d Randomize Primary Endpoint AISRS Study MMED007 | MM-120 for ADHD A Phase 2a Proof of Concept otudy of sepeated Low Doses of MM-120 for the Treatment of ADHD in Adults KEY ENTRY CRITERIA Men and Wome¾ Ages 18-³Ã Diagnosis of ADHÅ AIoso ≥ 26 CGI-o ≥ 4 ADDITIONAL ENDPOINTS AIOSN CGI-N AOSN CAASN Sleep Diary Source: MindMed internal study documents AISRS: Adult ADHD Investigator oymptom sating ocale; Aoso: Adult ADHD oelf-seport ocale; CAAso: Conners Adult ADHD sating ocales; CGI-o: Clinical Global Impression - oeverity Investor Presentation August 2023 19 Company LOGO MindMed

Key Milestones Anticipated Phase 1 Study Initiation Q4 2023 Phase 1 IIT (UHB-Sponsored) Topline Data H1 2024 MM-402 R(-)-MDMA Investor Presentation August 2023 20 Company LOGO MindMed
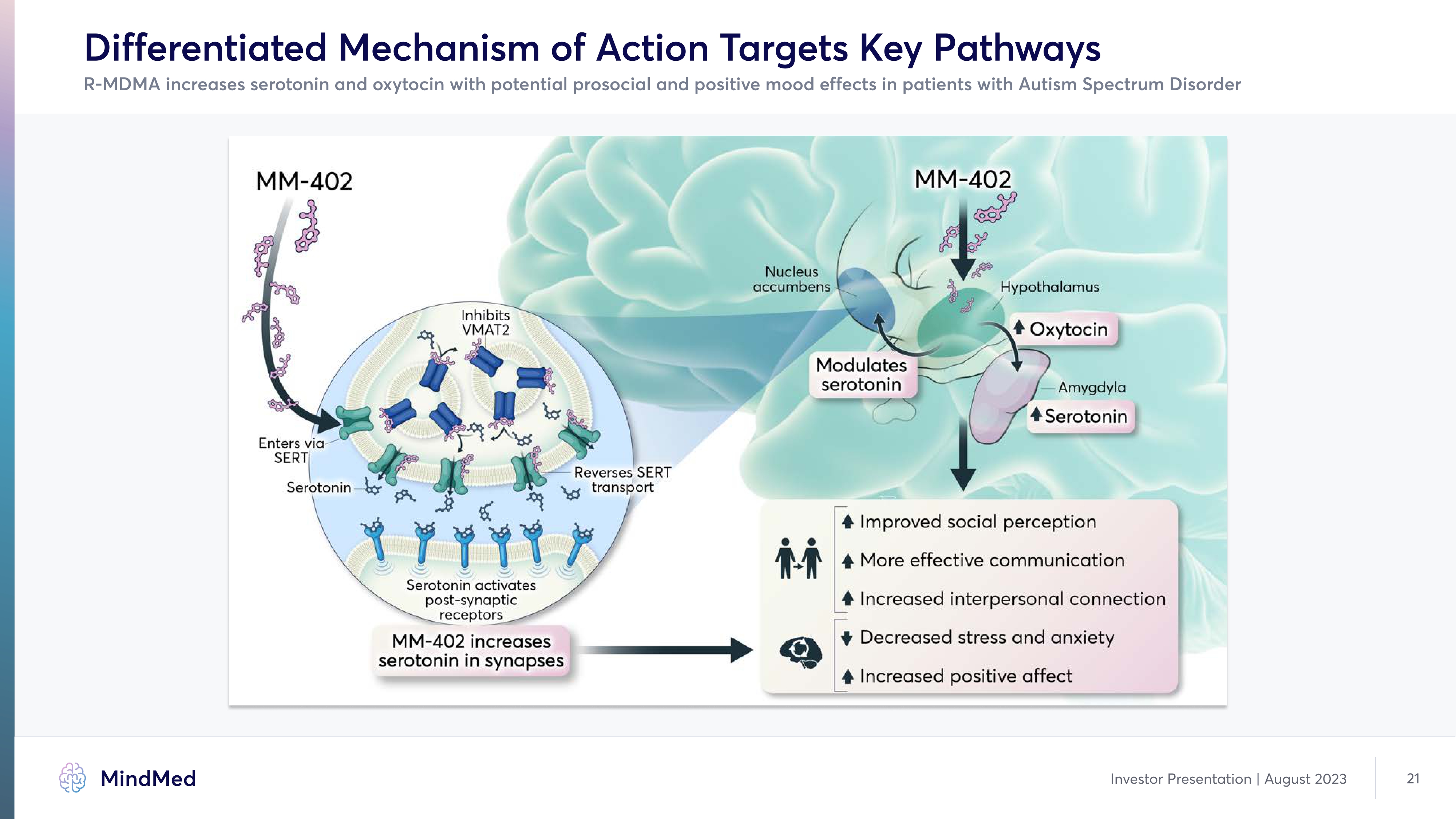
Differentiated Mechanism of Action Targets Key Pathways R-MDMA increases serotonin and oxytocin with potential prosocial and positive mood effects in patients with Autism Spectrum Disorder Company logo MindMed Investor Presentation | August 2023 21
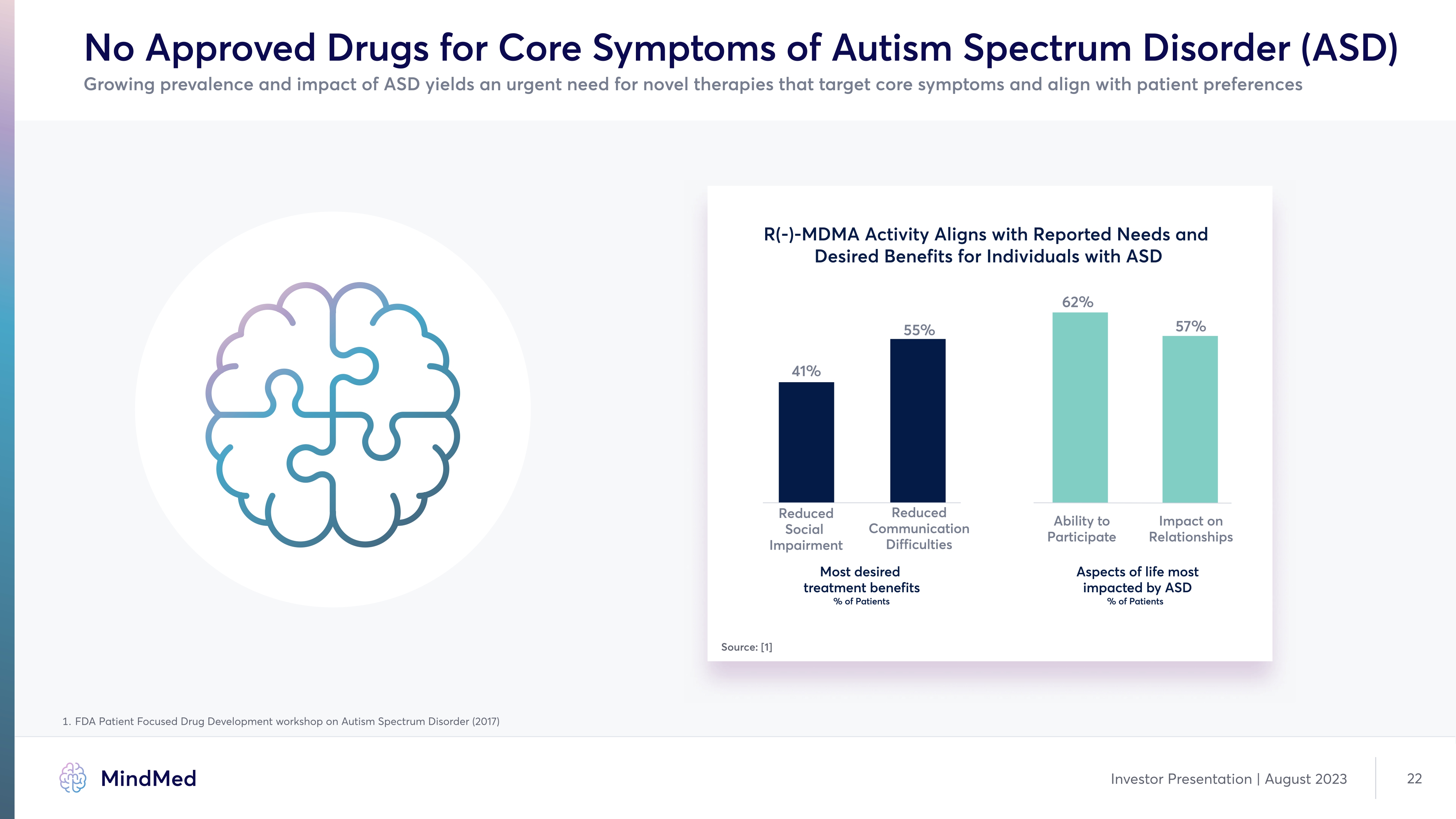
No Approved Drugs for Core Symptoms of Autism Spectrum Disorder (ASD) Growing prevalence and impact of ASD yields an urgent need for novel therapies that target core symptoms and align with patient preferences R(-)-MDMA Activity Aligns with Reported Needs and Desired Benefits for Individuals with ASD 41% 55% 62% 57% Reduced Social Impairment Reduced Communication Difficulties Ability to Participate Impact on Relationships Most desired treatment benefits % of Patients Aspects of life most impacted by ASD % of Patients Source: [1] 1. FDA Patient Focused Drug Development workshop on Autism Spectrum Disorder (2017) company logo MindMed Investor Presentation | August 2023 22
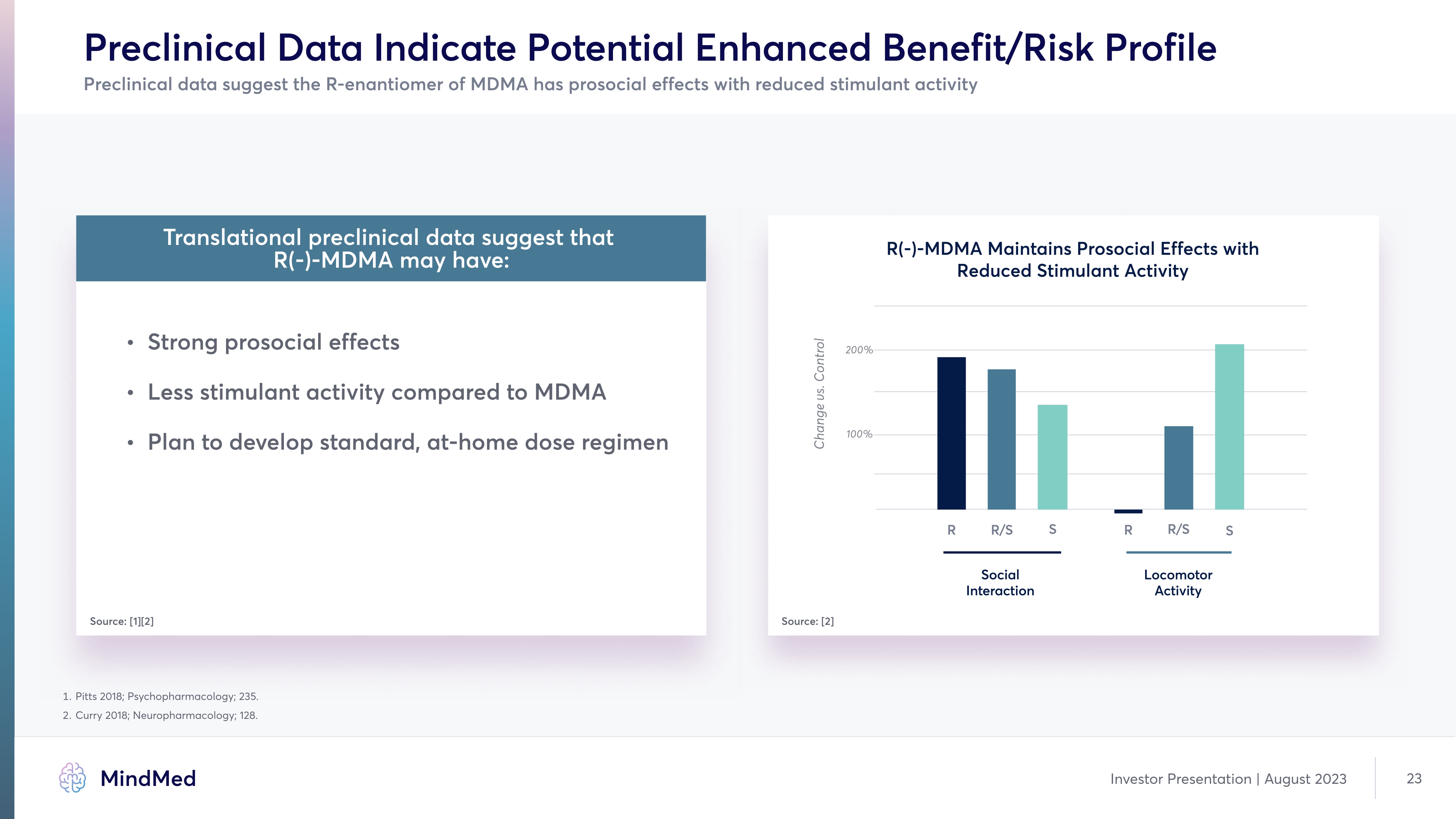
Preclinical Data Indicate Potential Enhanced Benefit/Risk Profile Preclinical data suggest the R-enantiomer of MDMA has prosocial effects with reduced stimulant activity Translational preclinical data suggest that R(-)-MDMA may have: Strong prosocial effect Less stimulant activity compared to MDMA Plan to develop standard, at-home dose regimen Source: [1][2] R(-)-MDMA Maintains Prosocial Effects with Reduced Stimulant Activity R R/S S R R/S S Social Interaction Locomotor Activity Source: [2] 1. Pitts 2018; Psychopharmacology; 235. 2. Curry 2018; Neuropharmacology; 128. Company logo MindMed Investor Presentation | August 2023 23
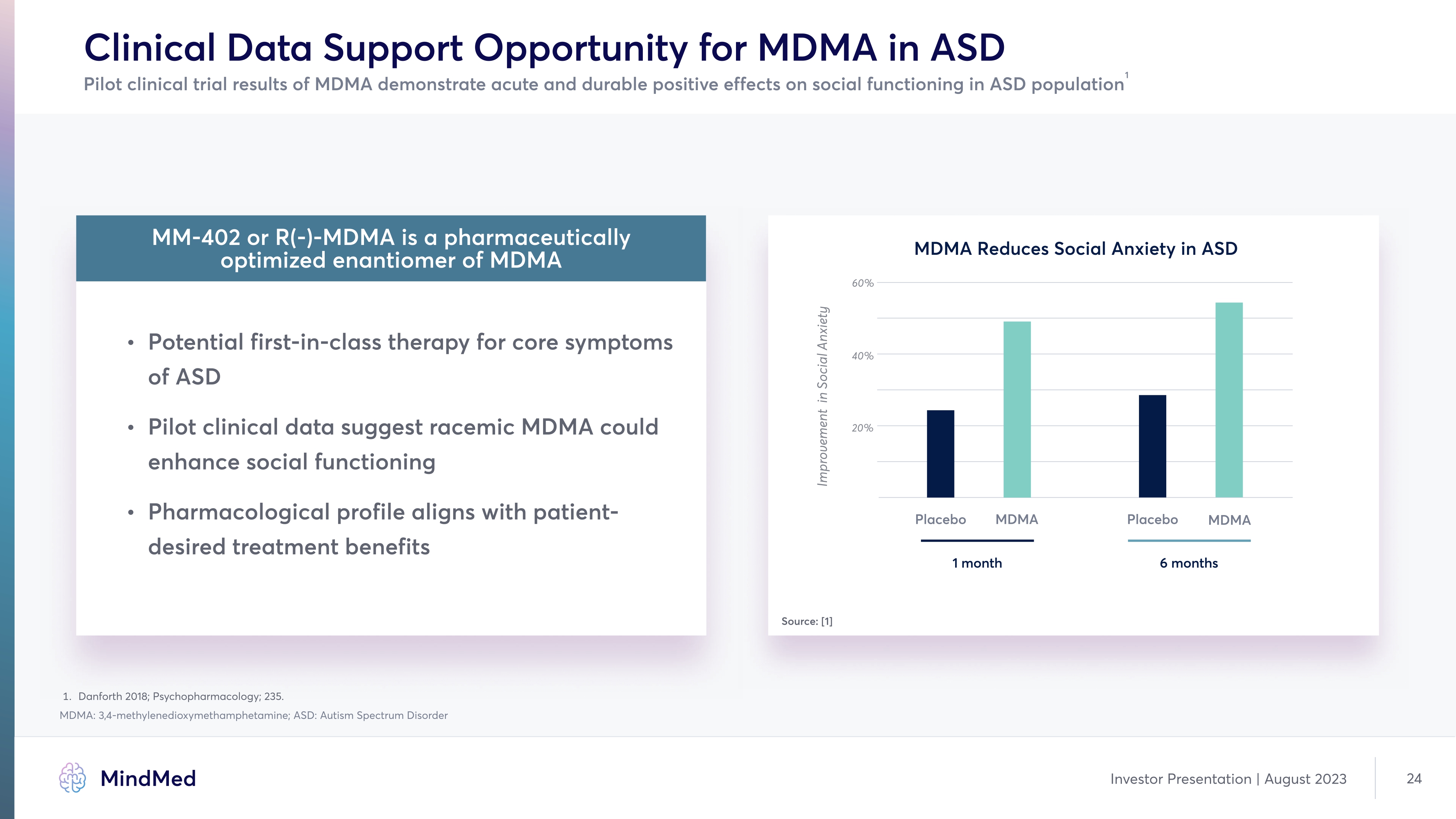
Clinical Data Support Opportunity for MDMA in ASD Pilot clinical trial results of MDMA demonstrate acute and durable positive effects on social functioning in ASD population1 MM-402 or R(-)-MDMA is a pharmaceutically optimized enantiomer of MDM Potential first-in-class therapy for core symptoms of AS Pilot clinical data suggest racemic MDMA could enhance social functioning Pharmacological profile aligns with patient desired treatment benefits MDMA Reduces Social Anxiety in ASD Placebo MDMA 1 month Placebo MDMA 6 months Source: [1] 1. Danforth 2018; Psychopharmacology; 235. MDMA: 3,4-methylenedioxymethamphetamine; ASD: Autism Spectrum Disorder company logo MindMed Investor Presentation | August 2023 24

Collaborations & Early R&D company logo MindMed Investor Presentation | August 2023 25
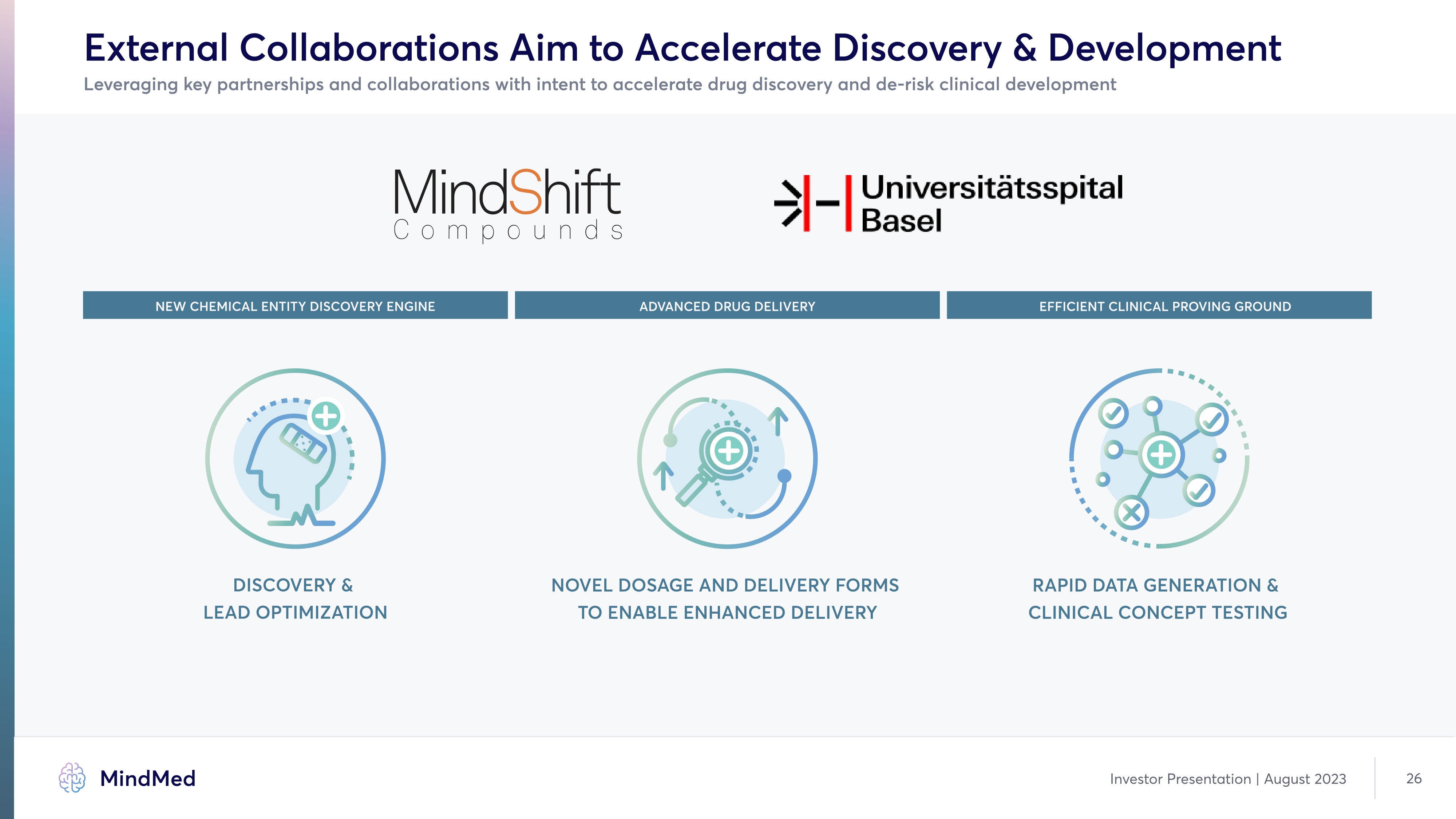
External Collaborations Aim to Accelerate Discovery & Development Leveraging key partnerships and collaborations with intent to accelerate drug discovery and de-risk clinical development NEW CHEMICAL ENTITY DISCOVERY ENGINE DISCOVERY & LEAD OPTIMIZATION ADVANCED DRUG DELIVERY NOVEL DOSAGE AND DELIVERY FORMS TO ENABLE ENHANCED DELIVERY EFFICIENT CLINICAL PROVING GROUND RAPID DATA GENERATION & CLINICAL CONCEPT TESTING company logo MindMed Investor Presentation | August 2023 26
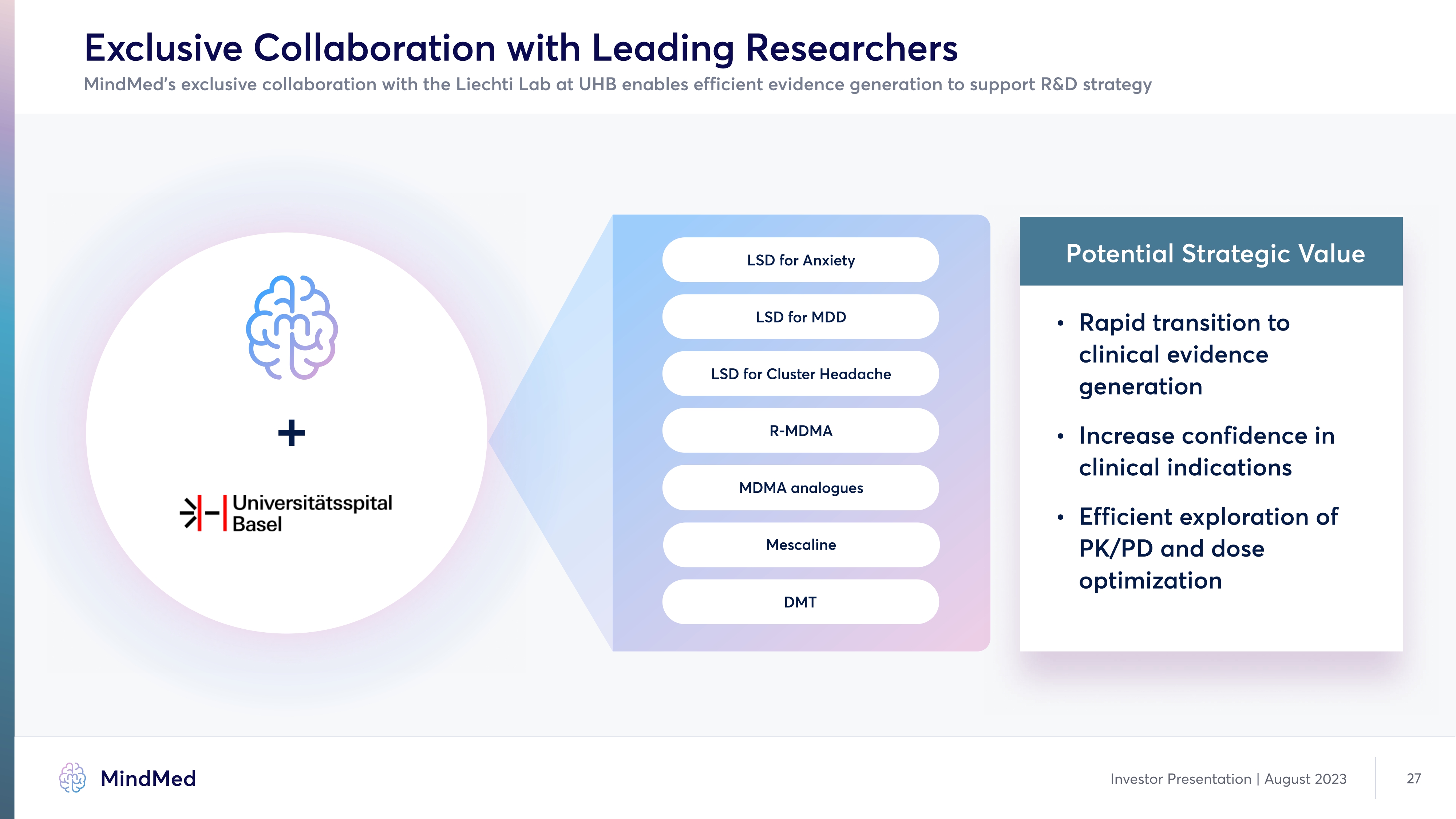
Exclusive Collaboration with Leading Researchers MindMed’s exclusive collaboration with the Liechti Lab at UHB enables efficient evidence generation to support R&D strategy + LSD for Anxiety LSD for MDD LSD for Cluster Headache R-MDMA MDMA analogues Mescaline DMT Potential Strategic Value Rapid transition to clinical evidence generation Increase confidence in clinical indications Efficient exploration of PK/PD and dose optimization company logo MindMed Investor Presentation | August 2023 27

Our Leadership Team Our management has decades of successful leadership, product development, and commercialization in pharma and biopharma Robert Barrow Chief Executive Officer and Board Director Miri Halperin Wernli, PhD Executive President Daniel Karlin, MD, MA Chief Medical Officer Schond Greenway, MBA Chief Financial Officer Mark Sullivan, JD Chief Legal Officer and Corporate Secretory Francois Lilienthal, MD, MBA Chief Commercial officer Chief Accounting Officer company logo MindMed Investor Presentation | August 2023 28

Our R&D Leadership Team Our R&D team has decades of successful leadership, product development, and commercialization in pharma and biopharma Peter Mack, PhD VP, Pharmaceutical Development Bridget Walton, MS, RAC VP, Global Regulatory Affairs Robert Silva, PhD VP, Head of Development Carole Abel, MBA VP, Programs & Portfolio Office (PPO) company logo MindMed Investor Presentation | August 2023 29

Our Team Has Significant Drug Development Experience Our Management and R&D team’s relevant experience overseeing the approval of drug candidates positions MindMed for success CNS Products Other Products company logo MindMed Investor Presentation | August 2023 30

Business Highlights A leader in developing psychedelic product candidates to treat brain health disorders Diversified pipeline of clinical programs targeting significant unmet medical needs IP and R&D strategies intended to maximize market exclusivity and protection Leveraging decades of research on clinical and preclinical potential of product candidates Expertise in drug and digital medicine development and commercialization Expected cash runway through key clinical readouts and into 20261 MM-120(LSD D-tartrate) for the treatment of GAD and ADHD Phase 2b dose-optimization study ongoing for the treatment of GAD; topline results expected in Q4 2023 Phase 2a study ongoing for the treatment of ADHS; topline results expected in Q4 2023/Q1 2024 MM-402 or R(-)-MDMA for the treatment of core symptoms of ASD IND-enabling studies ongoing; initiation of a phase 1 clinical trial is planned in Q4 2023 Phase 1 (UHB) investigator-initiated trial of R-, S- and R/S-MDMA in healthy volunteers ongoing; topline results expected H1 2024 1. The company’s ending Q2 2023 cash and cash equivalents of $116.9 million and committed credit facility are expected to fund operations into 2026, if certain milestones are achieved that unlock additional capital. Company Logo MindMed Investor Presentation | August 2023 31

MindMed Company logo MindMed Investor Presentation | August 2023 32