
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A
Proxy Statement Pursuant to Section 14(a) of the Securities
Exchange Act of 1934
Filed by the Registrant ☒
Filed by a Party other than the Registrant ☐
Check the appropriate box:
☐ Preliminary Proxy Statement
☐ Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2))
☐ Definitive Proxy Statement
☒ Definitive Additional Materials
☐ Soliciting Material Pursuant to §240.14a-12
Mind Medicine (MindMed) Inc.
(Name of Registrant as Specified In Its Charter)
N/A
(Name of Person(s) Filing Proxy Statement, if other than the Registrant)
Payment of Filing Fee (Check all boxes that apply):
☒ No fee required
☐ Fee paid previously with preliminary materials
☐ Fee computed on table in exhibit required by Item 25(b) per Exchange Act Rules 14a-6(i)(1) and 0-11
On May 31, 2023, Mind Medicine (MindMed) Inc. released an updated investor presentation in connection with its 2023 Annual Meeting of Shareholders.

Mindmed FCM’s Claims vs. Reality May 2023.

Disclaimer Mindmed Cautionary Notes and Forward-Looking Statements Certain statements in this presentation related to Mind Medicine (MindMed) Inc. (the “Company” or “MindMed”) constitute “forward-looking information” within the meaning of applicable securities laws and are prospective in nature. Forward-looking information is not based on historical facts, but rather on current expectations and projections about future events and are therefore subject to risks and uncertainties which could cause actual results to differ materially from the future results expressed or implied by the forward-looking statements. These statements generally can be identified by the use of forward-looking words such as “will”, “may”, “should”, “could”, “intend”, “estimate”, “plan”, “anticipate”, “expect”, “believe”, “potential” or “continue”, or the negative thereof or similar variations. Undue reliance should not be placed on forward-looking information, which are inherently uncertain, are based on estimates and assumptions, and are subject to known and unknown risks and uncertainties (both general and specific) that contribute to the possibility that the future events or circumstances contemplated by the forward-looking statements will not occur. There can be no assurance that the plans, intentions or expectations upon which forward-looking statements are based will in fact be realized. Forward-looking information in this presentation includes, but is not limited to, statements regarding the potential benefits and development of the Company’s product candidates, trials, studies and programs; the strengths and benefits of the Company’s strategic plan; the Company’s business plans and objectives; the ability of MindMed to achieve success consistent with management’s expectations; and the expected impact and results of the Company’s corporate governance practices, including of the Company Board’s director nominees. Forward-looking information is based on the opinions and estimates of management of the Company at the date the statements are made, as well as a number of assumptions made by, and information currently available to, the Company concerning, among other things, anticipated performance of its product candidates and programs, business prospects, strategies, regulatory developments, the development of its product candidates into effective products, the ability to produce products if approved, the approval by regulators of any products that are developed, and the non-occurrence of the risks and uncertainties outlined below or other significant events occurring outside of MindMed’s normal course of business. Although management of the Company considers these assumptions to be reasonable based on information currently available to it, they may prove to be incorrect.There are numerous risks and uncertainties that could cause actual results and the Company’s plans and objectives to differ materially from those expressed in the forward-looking information, including history of negative cash flows; limited operating history; incurrence of future losses; availability of additional capital; changes in market conditions; lack of product revenue; compliance with laws and regulations; changes in government policy; difficulty associated with research and development; risks associated with clinical trials or studies; heightened regulatory scrutiny; early stage product development; clinical trial risks; regulatory approval processes; novelty of the psychedelic inspired medicines industry; as well as those risk factors discussed or referred to herein and the risks described in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2022 and the Company’s Quarterly Report on Form 10-Q for the fiscal quarter ended March 31, 2023 under headings such as “Special Note Regarding Forward-Looking Statements,” and “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and other filings and furnishings made by the Company with the securities regulatory authorities in all provinces and territories of Canada which are available under the Company’s profile on SEDAR at www.sedar.com and with the U.S. Securities and Exchange Commission (“SEC”) on EDGAR at www.sec.gov. Except as required by law, the Company undertakes no duty or obligation to update any forward-looking statements contained in this presentation as a result of new information, future events, changes in expectations or otherwise. Additional Information and Where to Find It MindMed has filed with the SEC and Canadian securities regulatory authorities on May 1, 2023 a definitive proxy statement on Schedule 14A (the “proxy statement”), containing a form of WHITE universal proxy card, with respect to its solicitation of proxies for the annual general meeting of shareholders of MindMed on June 15, 2023 (the “Annual Meeting”). Details concerning the nominees of MindMed’s Board for election at MindMed’s Annual Meeting are included in the proxy statement. This presentation is not a substitute for the proxy statement or other document that MindMed has filed or may file with the SEC and Canadian securities regulatory authorities in connection with any solicitation by MindMed. INVESTORS AND SECURITY HOLDERS ARE URGED TO READ THE PROXY STATEMENT (INCLUDING ANY AMENDMENTS OR SUPPLEMENTS THERETO AND THE ACCOMPANYING WHITE UNIVERSAL PROXY CARD) FILED BY MINDMED AND ANY OTHER RELEVANT DOCUMENTS FILED WITH THE SEC AND CANADIAN SECURITIES REGULATORS WHEN THEY BECOME AVAILABLE CAREFULLY AND IN THEIR ENTIRETY BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT MINDMED AND ANY SOLICITATION. Investors and security holders may obtain copies of these documents and other documents filed with the SEC and Canadian securities regulatory authorities by MindMed free of charge through the website maintained by the SEC at www.sec.gov or through the Company’s profile on SEDAR at www.sedar.com. Copies of the documents filed by MindMed are also available free of charge by accessing MindMed’s website at www.mindmed.co.Participants in the Solicitation This presentation is neither a solicitation of a proxy or consent nor a substitute for any proxy statement or other filings that may be made with the SEC and Canadian securities regulatory authorities. Nonetheless, MindMed, its directors and executive officers and other members of management and employees may be deemed under U.S. securities laws and Canadian securities laws to be participants in the solicitation of proxies with respect to a solicitation by MindMed. Information about MindMed’s executive officers and directors and other participants in the solicitation, including their respective interests, by security holders or otherwise, is available in the proxy statement. To the extent holdings of MindMed securities reported in the proxy statement for the Annual Meeting have changed, such changes have been or will be reflected on Statements of Change in Ownership on Forms 3, 4 or 5 filed with the SEC and if applicable, on the System for Electronic Disclosure by Insiders (SEDI) in accordance with insider reporting requirements of Canadian securities laws. These documents are or will be available free of charge at the SEC’s website at www.sec.gov and either through the Company’s profile on SEDAR at www.sedar.com or updated filings on SEDI at www.sedar.ca.
Protecting MindMed Shareholders Mindmed MindMed is highly concerned by FCM’s continued willingness to mislead shareholders In its materials, FCM relies on cherry picked data, distortions and falsehoods to support why it should be given control of MindMed’s Board We believe that FCM has been willing to disregard the facts to such a blatant degree because: 1 FCM’s nominees are not qualified for MindMed’s Board – especially in comparison to our highly accomplished slate of directors 2 FCM’s ideas – particularly its ill-advised plan to “skip” Phase 2 trials for MM-120 – lack credibility, as multiple third party experts have now publicly stated 3. FCM’s campaign is not about what is best for MindMed or its shareholders. It is about turning back the clock on the Company’s progress and giving Scott Freeman influence by any means necessary. Consider – in FCM’s investor presentation, Scott Freeman is referenced 17 times,1 whereas all three of FCM’s other nominees are mentioned only seven times combined2 We want to correct the record around FCM’s deceptive statements and claims The choice is clear: support MindMed’s continued progress – not FCM’s misleading campaign 1,2. Excluding bio and legal pages.

FCM Blatantly Misrepresents the Qualifications of Our Nominees Mindmed FCM’S CLAIM REALITY Over a decade of experience leading drug development programs Industry-leading experience in clinical and regulatory strategy for psychedelics, particularly in psychiatry Extensive board and financial leadership experience Robert Barrow Chief Executive Officer Roger Crystal, MD Member of Comp Committee and Nom and Corp Gov Committee Led development and FDA approval of multiple CNS products (NARCAN® Nasal Spray; Opvee® - which was approved just last week) Physician with extensive R&D experience from preclinical through drug approval – including leading clinical trials for psychiatric therapies Significant commercialization experience Andreas Krebs Vice Board Chair, Chair of Nom and Corp Gov Committee, Member of Audit Committee Served as president and executive board member of Wyeth in the United States Primary responsibilities at Wyeth included global pricing and commercialization Former member of the Corporate R&D Executive Committee at Wyeth Carol A. Vallone Board Chair, Chair of Comp Committee, Member of Audit Committee Chair of the board of trustees for McLean Hospital, the #1 ranked psychiatric hospital in America and largest psychiatric affiliate of Harvard Medical School Extensive public board and corporate governance expertise in the healthcare industry CEO experience raising capital and scaling global companies with multiple successful exits FCM’s characterization of our directors’ skills and experience is false dishonest

FCM’s Cherry Picked Engagement Timeline Mindmed FCM’S CLAIM REALITY FCM’s distortions and omissions from its engagement with MindMed are too numerous to list, but include: At Scott Freeman’s request, the Board invited him to be part of the Nominating and Corp Gov. Committee’s process for selecting new directors in May 2022 – and Scott Freeman did not respond FCM’s aggressive and unprofessional public campaign beginning in August 2022: MindMed made numerous proposals to FCM to avoid a proxy contest – but ultimately FCM was unwilling to settle for less than 50% control of the Board, even though FCM only owns ~3.5% of the Company’s shares FCM distorts its engagement with MindMed in an attempt to appear reasonable – because the full facts tell a very different story misleading misleading

LOGO FCM is Trying to Rewrite the History of MM 110 FCM’S CLAIM REALITY DISTORTING Scott Freeman attempted to develop MM 110 (then called 18 MC) for approximately nine years before he and his co founder folded the program into MindMed A fter receiving adverse FDA feedback in 2014, Scott Freeman did nothing to address the FDA’s concerns with MM 110 As a result, flawed clinical and regulatory strategies adopted under his leadership ultimately led to the Board’s determination to reallocate resources away from the program Scott Freeman wants to blame MindMed for his own failures with MM 110 6
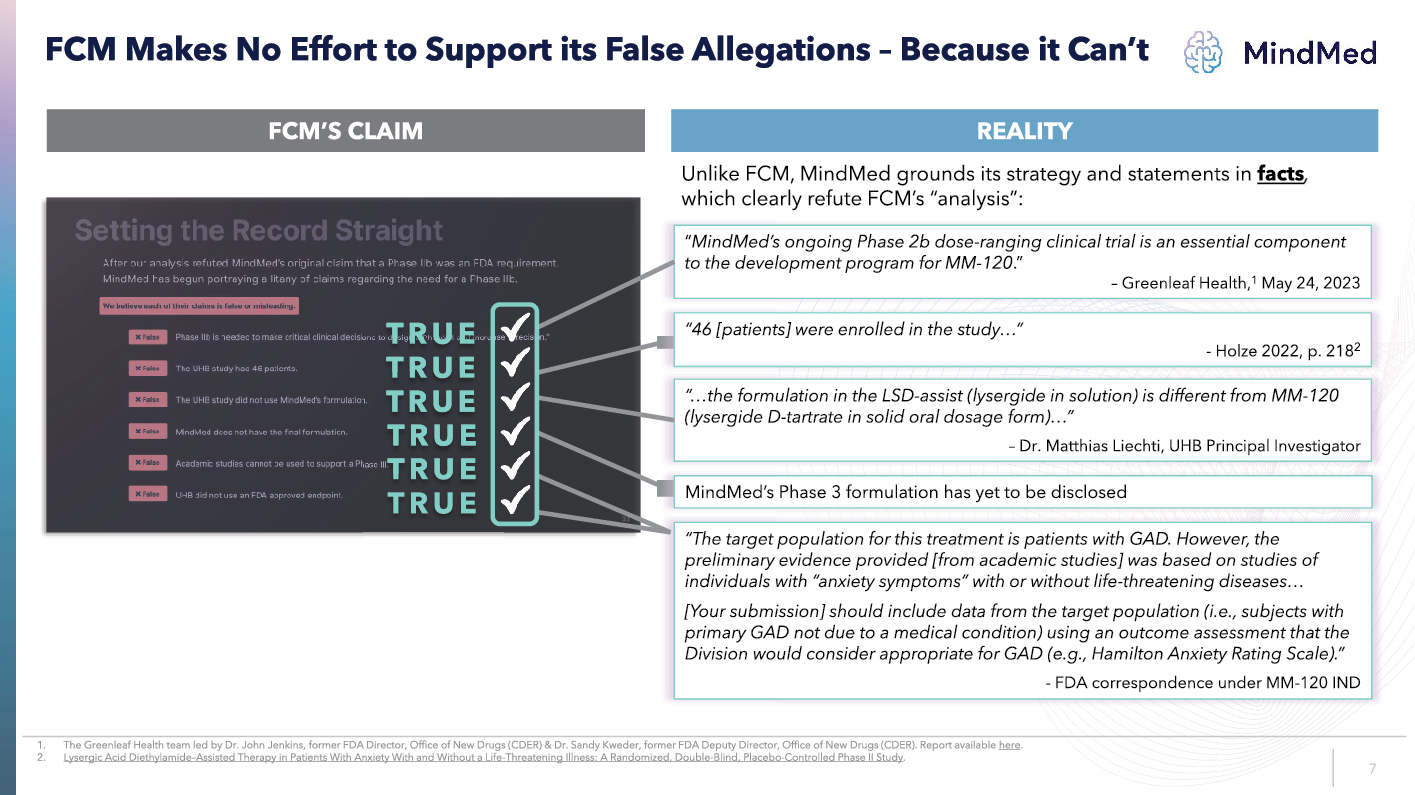
LOGO FCM Makes No Effort to Support its False Allegations Because it Can’t FCM’S CLAIM REALITY “…the formulation in the LSD assist ( lysergide in solution) is different from MM 120 lysergide D tartrate in solid oral dosage form)…” – Dr. Matthias Liechti , UHB Principal Investigator “The target population for this treatment is patients with GAD. However, the preliminary evidence provided [from academic studies] was based on studies of individuals with “anxiety symptoms” with or without life threatening diseases… [Your submission] should include data from the target population (i.e., subjects with primary GAD not due to a medical condition) using an outcome assessment that the Division would consider appropriate for GAD (e.g., Hamilton Anxiety Rating Scale).” - FDA correspondence under MM 120 IND “ MindMed’s ongoing Phase 2b dose ranging clinical trial is an essential component to the development program for MM 120 .” – Greenleaf Health, 1 May 24, 2023 TRUE TRUE TRUE TRUE TRUE TRUE “46 [patients] were enrolled in the study…” - Holze 2022, p. 218 2 MindMed’s Phase 3 formulation has yet to be disclosed Unlike FCM, MindMed grounds its strategy and statements in facts , which clearly refute FCM’s “analysis”: 1. The Greenleaf Health team led by Dr. John Jenkins, former FDA Director, Office of New Drugs (CDER) & Dr. Sandy Kweder, former FDA Deputy Director, Office of N ew Drugs (CDER). Report available here 2. Lysergic Acid Diethylamide Assisted Therapy in Patients With Anxiety With and Without a Life Threatening Illness: A Randomized, Double Blind, Placebo Controlled Phase II Study 7
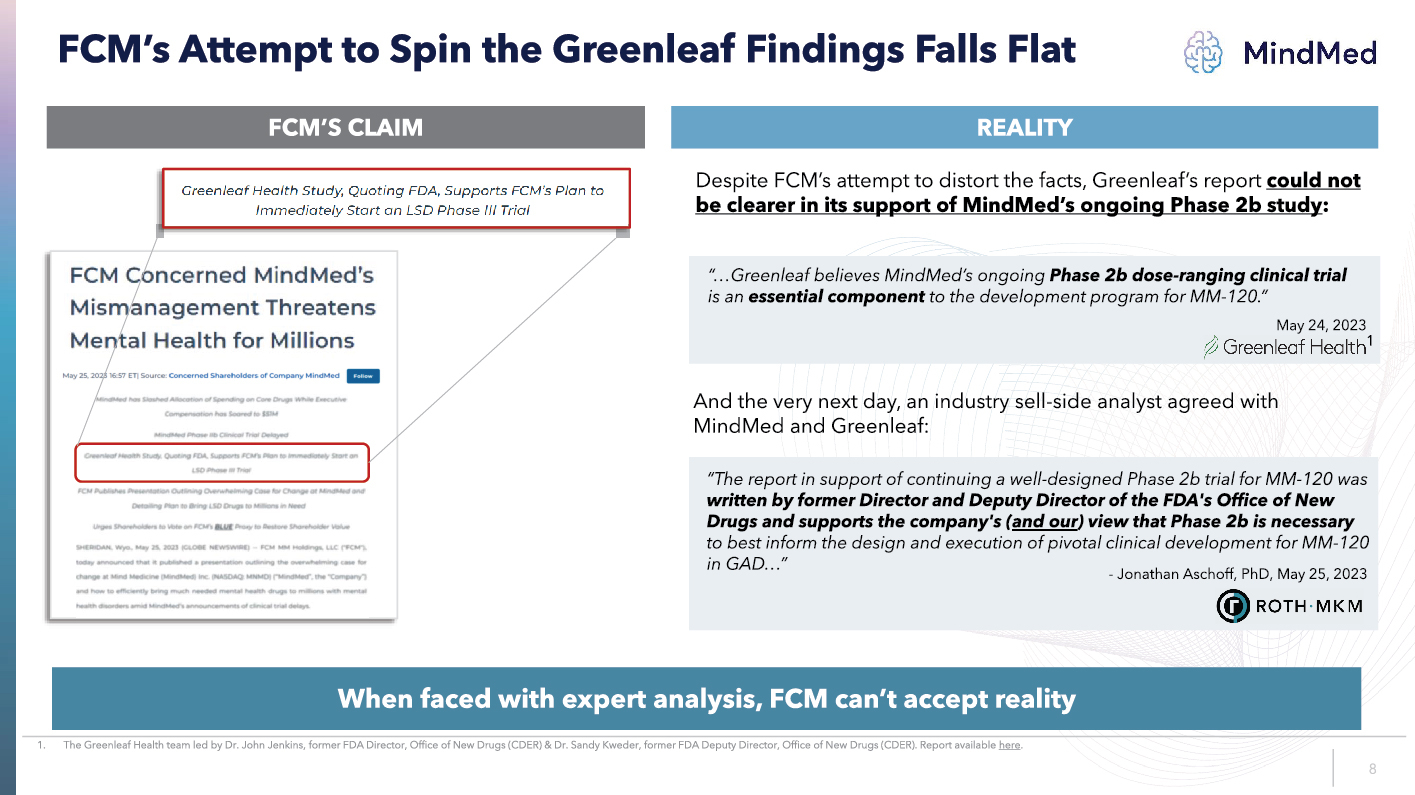
LOGO FCM’s Attempt to Spin the Greenleaf Findings Falls Flat FCM’S CLAIM REALITY Greenleaf health Study, Quoting FDA, Supports FCM’s Plan to Immediately start an LSD Phase III Trial FCM Concerned MindMed’s Mismanagement Threatens Mental Health for Millions And the very next day, an industry sell side analyst agreed with MindMed and Greenleaf: When faced with expert analysis, FCM can’t accept reality Despite FCM’s attempt to distort the facts, Greenleaf’s report could not be clearer in its support of MindMed’s ongoing Phase 2b study “…Greenleaf believes MindMed’s ongoing Phase 2b dose ranging clinical trial is an essential component to the development program for MM 120.” “The report in support of continuing a well designed Phase 2b trial for MM 120 was written by former Director and Deputy Director of the FDA's Office of New Drugs and supports the company's ( and our ) view that Phase 2b is necessary to best inform the design and execution of pivotal clinical development for MM 120 in GAD…” May 24, 2023 1. The Greenleaf Health team led by Dr. John Jenkins, former FDA Director, Office of New Drugs (CDER) & Dr. Sandy Kweder, former FDA Deputy Director, Office of N ew Drugs (CDER). Report available here 1 - Jonathan Aschoff , PhD, May 25, 2023 8
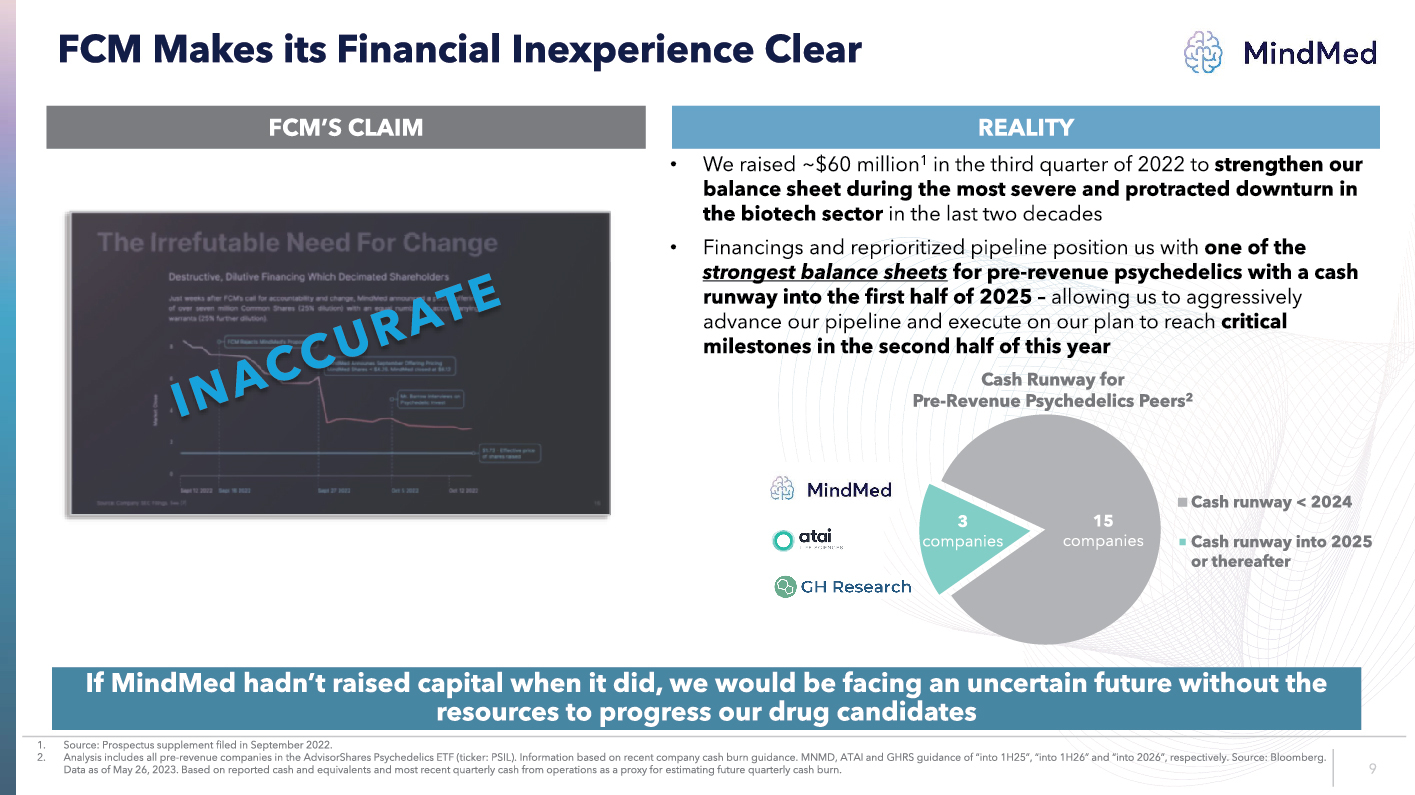
LOGO FCM Makes its Financial Inexperience Clear FCM’S CLAIM REALITY INACCURATE Cash Runway for Pre-Revenue Psychedelics Peers2 Cash runway < 2024 Cash runway into 2025 or thereafter If MindMed hadn’t raised capital when it did, we would be facing an uncertain future without the resources to progress our drug candidates We raised ~$60 million 1 in the third quarter of 2022 to strengthen our balance sheet during the most severe and protracted downturn in the biotech sector in the last two decades Financings and reprioritized pipeline position us with one of the strongest balance sheets for pre revenue psychedelics with a cash runway into the first half of 2025 allowing us to aggressively advance our pipeline and execute on our plan to reach critical milestones in the second half of this year 1. Source: Prospectus supplement filed in September 2022. 2. Analysis includes all pre revenue companies in the AdvisorShares Psychedelics ETF (ticker: PSIL). Information b ased on recent company cash burn guidance. MNMD, ATAI and GHRS guidance of “into 1H25”, “into 1H26” and “into 2026”, respecti vel y. Source: Bloomberg. Data as of May 26, 2023. Based on reported cash and equivalents and most recent quarterly cash from operations as a proxy for es timating future quarterly cash burn. 3 companies 15 companies 9

LOGO FCM’s Budget Would Put Achieving Key Milestones at Risk FCM’S CLAIM REALITY ? Relative to our closest sector peers, we spend materially less on SG&A in absolute and percentage terms and dedicate more of our resources towards R&D FCM’s budget seems based on nothing more than “spreadsheet math” and demonstrates FCM’s lack of real world experience The budget by FCM also shows a failure to understand the importance of having key personnel in place to successfully execute a Phase 3 program and scale a biotech company FCM’s naïve approach to budgeting for a public company could derail our progress 10
FCM Claims About Compensation Are Simply False FCM’S CLAIM REALITY MindMed’s executive compensation is directly linked to performance Over 80% of NEOs’ 2022 total direct compensation is structured to be “at-risk” with payout and value dependent company performance Realizable pay reflects the impact of stock performance: CEO’s 2022 realizable pay was nearly 80% less than reported pay1 Executive pay has not “soared” but instead trended dramatically down: total NEO reported pay is down 70% from 2021; equity grants to CEO in 2022 reflect a 62% decrease from 2021; CEO’s 2023 grants reflect a further 70% decrease 2022 executive pay was BELOW median of peers2 MindMed’s leadership is paid based on the Company’s performance in order to directly align their interests with those of shareholders Mindmed 1. “Realizable pay” is base salary, performance bonus earned and other compensation as reported in the Summary Compensation Table for 2022, but for equity awards granted during 2022, reflects the “intrinsic” value at the end of the year which is the value the award could deliver as of such time (ignoring vesting requirements) based on the stock price as of 12/31/22 of $2.20 USD. “Peers” in this slide refers to proxy compensation peer group developed by Compensation Committee with its independent compensation consultant for purposes of setting 2022 compensation.

Who Do You Trust? FCM Mindmed Unqualified nominees that do not possess the skills or experience to lead MindMed through this pivotal period A plan that has no credible basis, ignores multiple third party expert analyses and would expose the Company to significant risk A former co-founder who is attempting to turn back the clock and reset MindMed’s Board and strategy Directors with proven experience and expertise in the key areas of focus for MindMed A strategy that has seen positive momentum and positions MindMed for our first clinical trial data readouts later this year A team that is executing on MindMed’s plan to unlock value for patients and shareholders The choice is clear: Vote the White Card for all six of MindMed’s nominees

Vote the WHITE Universal Proxy Card to Protect MindMed Mindmed PROTECT YOUR INVESTMENT IN MINDMED Vote MindMed’s WHITE universal proxy card to vote “FOR” MindMed’s six highly qualified nominees, FOR the other proposals recommended by MindMed and WITHHOLD on FCM’s nominees www.ProtectMindMed.com INVESTOR CONTACT MORROW SODALI MEDIA CONTACT LONGACRE SQUARE PARTNERS Michael Verrechia / Eric Kamback MNMD@investor.morrowsodali.com Dan Zacchei / Joe Germani mindmed@longacresquare.com

Mindmed