UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A
(Rule 14a-101)
INFORMATION REQUIRED IN PROXY STATEMENT
SCHEDULE 14A INFORMATION
Proxy Statement Pursuant to Section 14(a) of the Securities Exchange Act of 1934
(Amendment No. )
Filed by the Registrant ☐
Filed by a Party other than the Registrant ☒
Check the appropriate box:
| ☐ | Preliminary Proxy Statement |
| ☐ | Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) |
| ☐ | Definitive Proxy Statement |
| ☒ | Definitive Additional Materials |
| ☐ | Soliciting Material Under § 240.14a-12 |
MIND MEDICINE (MINDMED) INC. |
(Name of Registrant as Specified In Its Charter) |
FCM MM HOLDINGS, LLC JAKE S. FREEMAN CHAD BOULANGER DR. SCOTT FREEMAN DR. FARZIN FARZANEH VIVEK JAIN ALEXANDER J. WODKA |
(Name of Persons(s) Filing Proxy Statement, if other than the Registrant) |
Payment of Filing Fee (Check all boxes that apply):
| ☒ | No fee required |
| ☐ | Fee paid previously with preliminary materials |
| ☐ | Fee computed on table in exhibit required by Item 25(b) per Exchange Act Rules 14a-6(i)(1) and 0-11 |
FCM MM Holdings, LLC, a Wyoming limited liability company, together with the other participants in its solicitation (collectively, “FCM”), has filed a definitive proxy statement and accompanying BLUE proxy card with the Securities and Exchange Commission to be used to solicit votes for the election of its slate of highly-qualified director nominees at the 2023 annual general meeting of shareholders of Mind Medicine (MindMed) Inc., a British Columbia corporation.
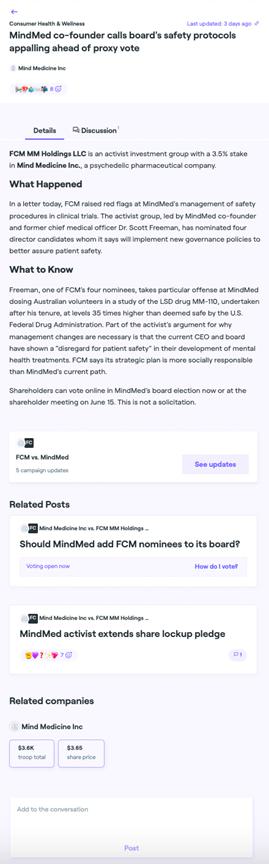
Item 1: On June 9, 2023, FCM posted the following materials on various social media platforms:

Item 2: On June 9, 2023, Dr. Freeman participated in a video interview with Public.com. The full version of the video interview was posted late June 9, 2023 and the full transcript of the interview, is pasted below.
All right.
0:05
Hi, everyone.
0:06
Thanks in advance for your time and interest and thanks for joining us.
0:09
My name is Felix Tabary, the CEO and co-founder at Troop. Troop is a community app for shareholder activism where everyday investors organize to change companies they own for good.
0:23
Today, I'm joined by an exciting guest, Scott Freeman, original co-founder of Mind Medicine, also known as MindMed, a New York based psychedelic medicine biotech company that develops psychedelic inspired medicines known as psychopathogens and therapies to address addiction and mental illness.
0:43
We're talking about LSD and mushrooms research here.
0:48
Today, we have an interesting situation.
0:51
Mind medicine.
0:52
Shareholders are coming together to make an important decision in the next couple days about who they would like to elect to the board of the company.
1:02
The election is an important one.
1:05
The board has an important role in the company.
1:07
And we're gonna hear a little bit today about Scott's background.
1:12
His experience co-founded the company's experience in this sector.
1:16
And then a little bit about what his recommendations are for the direction that the company needs to go in.
1:21
including the board nominees that Scott is advocating for without further ado I'm gonna turn it over to you Scott to tell us a little bit more about my med.
1:31
But one quick note, I do want to make, we do have one short disclosure.
1:36
So shareholders are being asked at this time to execute a proxy in favor of Scott Freeman and FCM’s nominees for the election to the board at the annual shareholder meeting.
1:48
And this message is sponsored by FCM which is the company that is behind the slate of board nominees up for election at Mindmed annual shareholder meeting on the 15th of June.
2:02
This message does not constitute financial advice.
2:06
Please do your own research and come to your own conclusions for more information, visit W W W dot troop dot com forward slash disclosures.
2:16
Once again, W W W dot troop dot com forward slash disclosures.
2:20
Thanks so much.
2:21
All right, Scott.
2:21
Well, welcome.
2:22
Thanks so much for joining us.
2:24
We'd love to hear a little bit about your background and your time in research and what led up to creating MindMed.
2:32
Well, thank you for having me as a guest.
2:35
So I'm a physician and I've worked in biotechnology for 25 years.
2:40
I developed drugs.
2:41
So I'm the person or the group of people who do the clinical trials of the human testing of the of, of of the drug to get it FDA approved.
2:52
And, I've done many trials, I've taken drugs to the FDA for approval.
2:59
I've gotten those drugs approved and probably about 12 years ago, started getting excited about the psychedelic space.
3:08
A lot had to do with looking at online surveys where people were reporting how great the drugs were working and how it was helping their disease.
3:18
And that's what we call anecdotal anecdotal data because it's really not well controlled and, and there's a lot of bias.
3:25
although there were a lot of people who were claiming benefit, so that's where I got really interested because it looked like the drug was working.
3:32
But until you really do what we call quote randomized studies where you compare the drug to what we call placebo, you really don't know that it really works and that this is all just, people taking the drug and we call it placebo effect thinking it works because they want it to work.
3:55
All right.
3:56
Thank you so much.
3:56
I appreciate it.
3:58
cool.
3:59
So, I guess one thing I'd, I'd love to learn a little bit more about is what it takes to, get one of these drugs to push through and to become available to a wider audience.
4:13
We'd love to learn a little bit more about.
4:16
I guess the, the FDA process, clinical trial process.
4:20
I know that these are, these tend to be slightly scary words.
4:22
And so, not a lot of people have a very strong understanding of them would love to turn it over to you if you could tell us a little bit more about that.
4:29
Yes, thank you.
4:29
And I think a lot of people hear the word FDA, including myself and get scared and, and, and believe it's a big bureaucracy.
4:38
and, and in some, in some cases, it, it is, but these are government, basically people, physicians and scientists that really are working to do what's in the, the public's best interest.
4:50
So I I I in general, they do, they do a a very good job.
4:55
However, there are obviously regulations and this, the process is, is long.
5:01
And so I think what we'll get into later is that time is of the essence, I mean, you have to develop drugs safely and efficiently, but you need to do it quickly because it's expensive amount of it, it's expensive to develop them.
5:13
And the more time you take to develop it, the more it costs and the less interest that investors have and for any drug to be successful, it often requires raising hundreds of millions of dollars.
5:26
And so you wanna help patients, but you also want to help the investors because you need them in order to get the money to develop the drug, to help, help, help the people.
5:38
So the FDA process is pretty typical for, for any drug you start with animal safety studies.
5:46
So you wanna know how safe and what doses, at least in animals are safe and you can use.
5:51
And from there it gives you some idea of what doses you can take into humans.
5:55
And you do what's called a phase one trial where you slowly increase the dose of the drug and look for toxicity effects.
6:02
So you start to understand what's a safe dose to give to a person.
6:08
And once you understand that safe dose, you phase two study, phase two study, you start treating patients with a disease, like as we'll talk about anxiety disorder or depression for, for LSD and you start looking for effects and there and once you see some effects, you then can go into a very large trial which call the phase three trial.
6:28
And that phase three trial randomizes people from against the drug and placebo and shows a statistically significant effect that the drug works and that leads to an FDA approval.
6:43
Understood.
6:44
Thanks so much for that context.
6:46
I guess it's a good way to segue into the next part of our conversation here.
6:51
So, Scott, when we introduced you, we talked about mind medicine, we kind of circled around or we've now kind of wrapped the topic of the FDA clinical trial process.
7:01
Could you help us get an understanding of what are the main drugs that mind medic is currently exploring.
7:07
And then if you could tie that back to where they're currently at in the development phase, be super helpful.
7:13
So, well, there were two drugs that we started with.
7:17
one's called MM110, which is a drug to treat opioid addiction.
7:22
And that drug actually was developed from a psychedelic drug that we dialed out the psychedelic effect.
7:30
And then the other drug and we'll get into a moment.
7:32
The difference between psy do drugs and nosy drugs and, and, and possibly why, why there's benefit for non psychedelic drugs.
7:40
And then there's a second drug MM-120 which is, which is called LSD.
7:45
And I think people are familiar with that and, and, and like I said, there was, there was a, there was a lot of data on online about surveys and, and we got interested and I think our, the interest was well founded.
8:02
Understood.
8:02
Thanks so much.
8:03
Let's actually dive into that for a second.
8:06
You mentioned MM-110, right?
8:08
which was originally conceived to try and treat opioid addiction.
8:13
Interesting point here about removing the psychedelic effect.
8:16
Tell me more about that.
8:19
So, to, to simplify it, I'll, I'll use LSD as the example.
8:23
So LSD, we're, we're doing two types of studies.
8:28
So one study is giving LSD at high doses.
8:33
But, but for a very limited time, so basically, let's say two doses, four weeks apart, but very high doses.
8:40
And, and we think that as you go to high doses of LSD, you get what's known as ego dissolution.
8:48
And and basically we believe that helps has the medicinal effect.
8:54
And the, but the other path for L S D is using low doses, what, what I think a lot of people may have heard of called micro dosing and they use about 25% of the dose or 20% of the dose twice a week for many months.
9:14
And so that we believe and, and also has benefit and you might ask, well, when do you use which one?
9:23
well, the high dose really, you wanna get that ego dissolution, you wanna get that, let's say blast effect to really change the disease.
9:34
And we have data that shows that that, that works in or general anxiety disorder and, and depression.
9:44
But then there's the other approach to micro dosing is where you're taking it longer term over time.
9:51
And you sort of, as I say, tickling the neurons long term to make that to, to get that effect, to start it and, and also lasting.
10:00
Although, the high dose effect seems to last too, so I think that depends, it's gonna depend on the disease.
10:09
And and a lot of other different factors on whether you would use the micro dosing.
10:16
And right now, my med has studies for microdosing in a DH D or you do that very high dose for a short period of time.
10:24
And that's a study that my med is looking at in general anxiety disorder.
10:32
Gotcha.
10:33
So speaking of general anxiety disorder, my understanding right now is that that, that is the, the core focus of the studies surrounding MM-120.
10:42
Is that correct?
10:44
Yes.
10:44
And so I think that's as I started this, you know, it's very expensive, it takes time to get drugs approved.
10:53
And so you need to have some focus and you need to take your lead and, and, and the lead has been MM-120 in General Anxiety disorder because we have a collaborator in University Hospital Basel who had done two randomized studies that showed L S D worked in General Anxiety Disorder.
11:17
And these were studies where that were randomized.
11:20
So one group of patients got L S D and another just got placebo.
11:25
And in both the studies, the L S D group had a very strong response, the L S D group, the, the, the the patient said that both of them had general anxiety, but the patient with general anxiety had a very strong response where their anxiety went down.
11:41
Whereas the placebo did not.
11:43
So from there, we're saying this is a drug you really want to move forward and, and, and develop and, and as I said, you want to develop it quickly but safely and we had the phase one data and, and now two randomized phase two studies.
12:02
So then, so, so, so then where is the issue?
12:06
Where is the, where's the contention?
12:09
Where's the blockage?
12:11
I think this is a good opportunity to kind of come into where there are different di differing points of view on, on, on the current situation.
12:19
Can you tell us a little bit more about what your perspective is on, you know, budgeting priorities and financing when it comes to this, this, this clinical trial phase?
12:28
What's your view on the direction that mind me should go in and what aren't they doing?
12:34
And what's the fix?
12:35
Yeah, so good question.
12:37
And as as was mentioned, we're in the middle of a proxy campaign and, and I wanna bring back some of myself, one of the original founders and, and some other directors to, to basically change the direction of the company.
12:55
I mean, there's, there's two parts to this one is that they're taking a lot of time, the clinical trials are delayed because they're I believe mismanaged, they're also have some regulatory flaws that they're testing the, the wrong dose regimen.
13:14
So the phase two studies that showed that L S D is effective at high doses that was given at for two doses each 14 weeks apart.
13:24
So you get a high dose, you wait four weeks to give it another high dose.
13:27
The current mind med management is looking at a one dose regimen but they have no data, no randomized phase two data to show that one dose is as good as two doses.
13:40
So what's the problem with that?
13:41
The problem is is that the next step is a phase three trial.
13:46
And our proposal is to use what we already know works two doses of L S D.
13:53
They're proposing one dose.
13:55
The problem is phase three trials are very expensive.
13:58
They cost 100 to $200 million and you only get one chance to do a phase three trial because if it fails, you can't go back to investors and say, oh, you know something, I think if I gave two doses, it would have worked.
14:10
I only gave one.
14:12
So that's one of the big issues.
14:15
I've developed drugs for 25 years.
14:19
I understand how to look at phase two data and go to a phase three trial.
14:24
The current management has really no drug development.
14:26
No, no drug development experience.
14:28
The, the chief medical officer has a background in bioinformatics.
14:33
He's never taken the drug to market.
14:35
The other issue is that, you know, they're overspending.
14:38
they've been, I look at waste wasting money.
14:42
The, the current management are not shareholders, they only own about 0.2% of the company.
14:47
I own 3%.
14:49
So the point being is that they're not aligned with shareholders, they're looking to, you know, for the most part, get their salaries and do their job and if it doesn't work, I mean, they don't, they don't, lose share value.
15:03
Right.
15:04
I do if I, if, if it doesn't work and that also leads to excessive spending and, and blow the cost, their, their current budget is 66 million.
15:13
And we think, and I've, I've worked at lean efficient biotechnology companies that have half the people that they have and that's what we're proposing to, cut, cut costs and cut personnel.
15:27
As many well managed companies are doing in these macroeconomic times where it's a, it's a bad market and, you have to be more efficient.
15:36
So our goal is to make the company more efficient, save money that will allow us to keep money longer.
15:43
So we don't have to raise money and dilute shareholders, but also quickly get a phase three trial started with the right dose regimen.
15:53
Gosh, I appreciate that.
15:54
I think.
15:55
it's, it's pretty clear what some of the objectives are.
15:58
Let's actually dig into that a little bit.
16:00
just by way of background.
16:02
11 thing typically that shareholders are concerned about when they hear of an outside party.
16:12
Well, in this case, really an inside outside party, you're already a shareholder in the company, you co-founded it.
16:18
One thing that shareholders tend to worry a little bit about when they see an outside party coming in with proposals to change the company, to improve it, to, to, to, to, to, to make it better.
16:30
Is that they tend to be in it typically for the short term looking for short term gains.
16:36
But you've communicated not just once but twice now that you are interested in locking up your shares.
16:45
What does that mean?
16:46
Why does that matter?
16:48
And why is that a strong argument here?
16:51
Yeah, that's a good question.
16:52
And we, we've made a big point about this.
16:55
It's, and I'll say it's unusual that, that, that people like myself do this, but I have, as I said, about 3% ownership in the company and I believe that if you invest in me, I have confidence in my plan and confidence in my work and that investors should profit first perform it.
17:18
So I'm committed to sell none of my shares.
17:22
At first, we came out for two years, but now we've increased as the next three years.
17:27
I will not trade a single share or sell any of my shares of my medicine.
17:32
Be partly so that, you know, whoever in invest in the company or invest or, or votes for us, they will be able to profit first.
17:41
But the second part is I believe in the long term future, the company under the man, my management and my other director candidates that in three years, this company will be much more valuable because we're gonna start the phase three which this company has delayed starting for the last three years, right?
17:59
So we're gonna start a phase three by the end of December, the December 2023.
18:05
And that will take three years to complete and get the drug approved.
18:09
So we anticipate getting this drug approved in 2026 both to help patients with, with mental mental health disorders, but also will help, will help investors.
18:20
The current management, their plan is, is probably because they haven't announced even when they're gonna start it, it's probably gonna be at least six years before they get a drug approved, which I think hurts both the patients and the investors.
18:35
So we're committed.
18:37
We stand by stand behind our work.
18:40
All the directors are locking up their shares, meaning we, we can't sell until for another three years until we show that our plan to bring LSD to the market in three years.
18:51
Yeah, phase three completed has happened.
18:56
Understood, I guess one quick one follow up question that I have here is you mentioned the other directors it's awesome to hear that there's a pledge here to lock up shares, there's alignment across the entire slate that's up for election.
19:10
Could you tell us just a little bit more about the nominees that you're advancing for the board?
19:18
Yeah.
19:19
So I think there's, there's two parts to it.
19:22
So one part that's significantly lacking at my medicine is clinical development experience.
19:28
And like I said, I have 25 years of clinical development development experience.
19:33
One of the other directors, Doctor Far and Farzana, he also has decades of clinical development experience.
19:40
So between both of us, we want to bring that level of experience to get these drugs FDA approved.
19:49
I mean, the other thing I'll, I, I'll, I'll point to is and why it's, it's also important to get this phase three started and the drug approved in 2026 we put out a press release and stated that there's a company called Maps that's developing MDMA for post traumatic stress syndrome and they plan on filing their drug in the end of this year, 2023 with the FDA.
20:19
And I've looked at the data and their data is similar to LSD.
20:22
That's another reason why I think LSD is really good and is gonna get approved.
20:27
But I also think MDMA is gonna get approved by mid 2024 and that will open the door at the FDA. So with any new technology, usually the FDA’s the doors closed a bit until they really understand it and feel comfortable with technology.
20:43
I believe that 2024 mid 2024 the psychedelic revolution is gonna start and, and that's gonna start with MDMA getting approved for PTSD.
20:54
When that happens, the next group of drugs that come through that door, there's usually a little bit lower bar and so it's, it's, let's say, I, I'd say easy to approve but, but the FDA wants to get these drugs out.
21:11
What then happens in the next 34 or five years is all companies are rushing that open door and then either for time constraints and, and resources, the bar gets higher.
21:25
So one of the reason, the other reason why besides getting this to patients quickly to get this case to be started and completed by 2026 is we wanna be able to walk through that door early.
21:36
And so I think that's why having clinical development experience between myself and far and far is important.
21:43
The other two candidates states, Alex Wonka and Vivek Jane have financial background experience.
21:50
So the other problem with the company is that is that they're wasting money.
21:56
I mean, so they, they're gonna run out of money according to the analysts by mid 2024 although they keep telling everybody it's gonna last at 2025.
22:08
So they're trying to pull the wool over people's eyes and we're sort of against that.
22:12
It's very clear that they're, they're running out of money.
22:15
they have 100 $30 million in the bank.
22:18
So, what I want to have is good financial people on board.
22:21
Also, as we go through our cut costs, cutting costs, we wanna keep that money so that we don't have to raise money in 2024.
22:30
But that money can last us to 2026 when the drugs finishing phase three and looking to get that approved because that's a value event and that's the time to raise money, particularly in these tough economic times.
22:44
So we really want to conserve cash.
22:46
As I said, the current management, they're not shareholders, they own 30.2%.
22:53
They're happy to just keep spending money and raising more money and taking big salaries.
22:58
We wanna come in, cut salaries, cut costs, extend the cash, get the phase three trust started.
23:05
So we're ready when the FDA opens the door in 2026.
23:08
And that will be a value driving event and probably a good time to raise money.
23:14
Awesome.
23:15
Thanks for that.
23:15
I really appreciate it.
23:17
And the message is super clear, I think to any shareholders listening to this message, one logistical operational point.
23:25
If you are a shareholder of mine Medicine, you have until I believe 11:59 PM on the 12th of June to make your decision.
23:35
Once again, thank you so much for having joined us.
23:38
Scott Freeman is here, co-founder of Mind Medicine, advocating for the four candidates for the board up for election at Mind Medicine out of the six on the slate.
23:49
Keep an eye out for team FCM’s nominees.
23:54
One quick statement here.
23:56
This is not a solicitation.
23:58
Please do your own research.
23:59
This is also not financial advice.
24:02
Most importantly, thank you so much to Katie and the team over at public for inviting us in over here to interview Scott and given the opportunity to kind of share his side of the story for the upcoming annual board member election.
24:17
It is incredibly important to vote your shares as a shareholder.
24:20
And just a reminder when you are a shareholder of a company, you have the right to vote typically once a year on who is on the board of the company and a handful of other important matters.
24:32
Thanks so much for having joined us for more information.
24:37
What the point.
24:38
I, I think that's correct.
24:39
You, you, you should vote.
24:41
I mean, this is a, a critical time in MindMed's future.
24:46
You know, they're running out of cash.
24:48
The phase three trials, this is, you know, we have different opinions.
24:53
I, you know, as I said, my opinion, I stand by it.
24:56
I'm locking up my shares.
24:58
I think the company needs to get back on track.
25:00
We need to preserve cash and get a phase three done, but you need to vote because, the, the, the, the company's future is at stake here.
25:10
Absolutely.
25:12
Absolutely.
25:12
Thanks so much for that.
25:13
It was a pleasure having you today.
25:15
Thanks so much for, for, for your time if you'd like more information about what's going on, go to troop dot com forward slash disclosure.
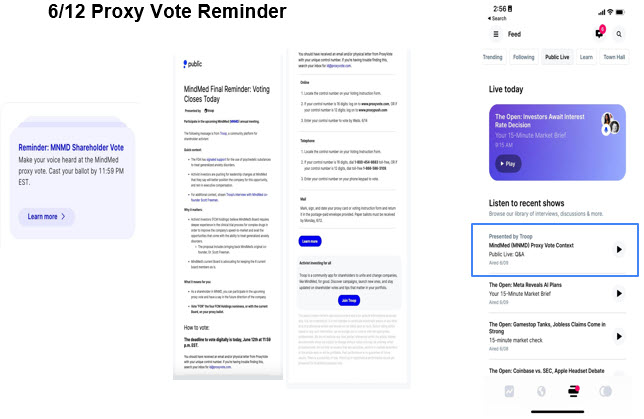
Item 3: On June 12, 2023, FCM posted the following materials on various social media platforms:
| Portfolio Reminder | Podcast Library |